Abstract
Studies of de novo cytokinin biosynthesis in isopentenyltransferase (ipt)-transformed Arabidopsis thaliana, involving in vivo deuterium labeling and mass spectrometry, showed that the biosynthetic rate of zeatinriboside-5′-monophosphate was around 66-fold higher than that of isopentenyladenosine-5′-monophosphate (iPMP), the proposed primary product of the Agrobacterium ipt. Double tracer analysis, using [2H6] isopentenyladenosine and deuterium oxide, provided evidence for an alternative, iPMP-independent, biosynthetic pathway for zeatin-type cytokinins, present in both ipt-expressing and wild-type Arabidopsis thaliana. Reduction of the biosynthetic flux in the alternative pathway by use of mevastatin, an inhibitor for 3-hydroxy-3-methylglutaryl CoA reductase, indicated a terpenoid origin for the side-chain precursor of the iPMP independent pathway.
Cytokinins are an important class of plant growth regulators, defined by their ability to promote cell division in tissue culture in the presence of auxins (1, 2). Virtually all naturally occurring cytokinins identified to date are adenine species substituted at N6 with an isoprenoid or aromatic side chain. In this text, cytokinins will refer solely to the isoprenoid cytokinin bases and their sugar conjugates. Cytokinins affect many plant developmental processes including cell division, cell differentiation, chlorophyll senescence, and apical dominance (3).
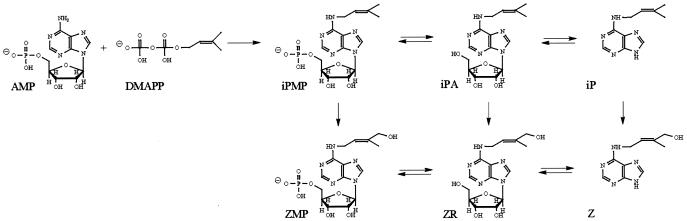
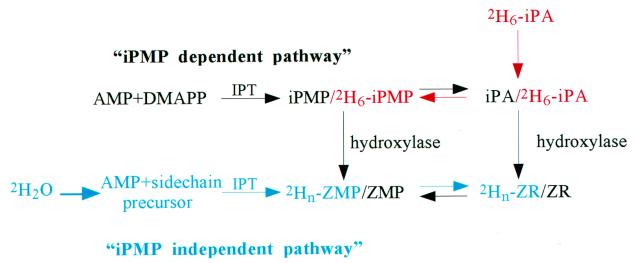
Efforts to elucidate the biosynthetic origin of cytokinins in plants have been inconclusive. Early suggestions that tRNA degradation could be the major source of free, active cytokinins (4) were disproved when calculations of tRNA turnover rates showed that a tRNA-independent de novo biosynthetic pathway also must be present in plants (5). A major breakthrough was the discovery of a cytokinin biosynthetic enzyme in the slime mold, Dictyostelium discoideum (6). Cell-free extracts from this organism can convert AMP and dimethylallyl-pyrophosphate (DMAPP) to the free cytokinins isopentenyladenosine- 5′-monophosphate (iPMP) and the corresponding nucleoside (isopentenyladenosine, iPA). This finding, and studies on the metabolism of isopentenyl-type cytokinins (7, 8), led to the proposal that iPMP is also the primary cytokinin intermediate in plants, and zeatin cytokinins are formed by hydroxylation of iPMP and its derivatives (9, 10) (Fig. 1).
Figure 1.
The established model of de novo cytokinin biosynthesis in plants with the formation of iPMP as the first cytokinin. iP, Isopentenyladenine; Z, zeatin.
Later, the product of the T-DNA gene 4 (ipt) of the crown gall-forming bacterium, Agrobacterium tumefaciens, also was described as a DMAPP:AMP isopentenyltransferase (ipt) (11, 12). AMP was found to be the preferred adenylic substrate for the Agrobacterium ipt enzyme but searches for alternative side-chain donors have been limited. However, when de novo biosynthesis in crown gall tissue of Vinca rosea was traced with 14C-adenine, in vivo production of iPMP was undetectable, whereas zeatinriboside-5′-monophospate (ZMP) production was strong (13). After studies of 14C-isopententyladenine metabolism in this system, it was proposed that the very low levels of iPMP were caused by rapid turnover of the iPMP pool (14). An enzyme, capable of catalyzing stereo-specific hydroxylation of the side chain, detected in microsomal fractions of cauliflower, Brassica oleracea, and metyrapone, a cytochrome P450-specific inhibitor, blocked this reaction (15). However, mass spectrometric analysis of the cytokinin contents of ipt-expressing plants revealed that levels of zeatinriboside (ZR) and ZMP tend to increase, whereas those of iPMP are unaffected (16) or even decrease, compared with wild-type plants (17), conflicting with the proposal that iPMP is the first product of the ipt gene. The lack of iPMP accumulation in ipt-transgenic plants was again explained by rapid conversion of iPMP to ZMP, together with rapid oxidative cleavage of the isopentenyl side chain by the action of cytokinin oxidase (16, 18). This enzyme shows a stronger affinity for isopentenyl-type cytokinins than the zeatin type, and it is induced by cytokinin overproduction (19, 20).
We have tested the proposed biosynthesis route by using an inducible cytokinin production system, to limit the possibility of interference from mechanisms initiated by high cytokinin concentrations that could inhibit iPMP accumulation. If such mechanisms exist in planta, then plants expressing the ipt gene under the control of an inducible expression system should show a transient accumulation of iPMP, after induction, before the putative iPMP homeostatic mechanisms are initiated. Therefore, we have analyzed cytokinin biosynthesis in transgenic Arabidopsis, expressing the ipt gene under the control of a glucocorticoid inducible transcription system (21, 22). This system recently was shown to cause growth defects in transgenic A. thaliana plants grown in inductive conditions for 1–2 weeks (23). However, our investigation is based solely on samples taken in the initial induction phase during which no effect from the long-term growth alteration is expected. We have previously developed powerful tools for analyzing cytokinins by mass spectrometry (24) and for quantitative analysis of cytokinin biosynthesis by in vivo deuterium labeling (25). In this paper we present evidence, acquired by using these techniques, for an alternative, iPMP-independent, biosynthetic pathway of zeatin-type cytokinins, operational in both wild-type and ipt-transgenic plants.
Materials and Methods
Plant Material and Growth Conditions.
The plants used in this study were of wild-type A. thaliana ecotype Landsberg, and a line (3–2) transformed with the A. tumefaciens ipt gene under the control of a glucocorticoid inducible expression system in pMON721 vector (21, 22). For Northern analysis and measurement of cytokinin induction, transgenic Arabidopsis plants were grown under sterile conditions on Murashige-Skoog medium, 0.8% agar plus 3% sucrose. For tracer experiments, transgenic Arabidopsis plants were grown on liquid Murashige-Skoog medium with 3% sucrose in 250-ml flasks, each containing 50 ml of medium and 25 plants (agitated, at 22°C, with an 18 h photoperiod, photon density 130 μE m−1⋅s−1).
Analysis of Induced Cytokinin Overproduction.
Four-week-old plants were sprayed with a solution of 30 μM dexamethasone in 0.02% Triton 100, and samples were harvested 0, 0.2, 0.5, 1, 2, 6, and 12 h after induction. Six cytokinins (the isopententenyl and zeatin bases, nucleosides, and nucleotides) were purified and analyzed by capillary-liquid chromatography/frit-fast atom bombardment-MS in selective reaction monitoring mode according to Åstot et al. (24). In a second set of experiments, the cytokinin nucleotide levels were quantified in plants grown and induced in liquid growth media free from tracers.
In vivo Labeling Experiments.
Three-week-old plants were transferred to 1/2 Murashige-Skoog media/1.5% sucrose containing 30% 2H2O, or 30% 2H2O combined with 1 μM [2H6]iPA. To induce ipt expression 30 μM dexamethasone was added. Metyrapone (2 and 5 mM) was used in cytochrome P450 inhibition experiments with wild-type and ipt-expressing plants, respectively. 3-Hydroxy-3-methylglutaryl (HMG) CoA reductase, catalyzing the formation of mevalonic acid, was inhibited by addition of 10 μM mevastatin. In this experiment, 2 mM metyrapone also was added to uncouple the iPMP-independent pathway. After 3–12 h of incubation, samples were harvested, and mass isotopomer analysis was performed by capillary-liquid chromatography/frit-fast atom bombardment-MS in high-resolution selected ion monitoring mode (ipt) and selective reaction monitoring mode (wild type) according to Åstot et al. (25).
Results
Induced ipt Expression and Cytokinin Overproduction.
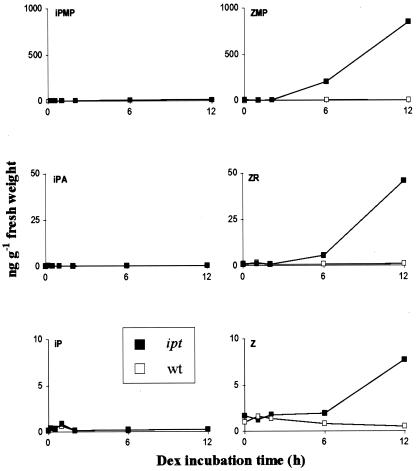
Significant increases in ipt transcript levels were detected 2–3 h after adding dexamethasone, a synthetic glucocorticoid, and levels were very high after 12 h incubation, similar to the induction kinetics in tobacco described by Aoyama et al. (21). These increases were followed by significant rises in concentrations of the zeatin-type cytokinins (Fig. 2). The ZMP level was most affected, rising to 850 ng⋅g-1 fresh weight after 12 h of induction: 100-fold higher than in uninduced plants. The ZR and zeatin levels rose 50- and 5-fold, respectively, during the same period of induction. In contrast to the zeatin family, the iPMP, iPA, and isopententyladenine pool sizes were not significantly affected by induction of the ipt gene. When ipt expression was induced for 12 h in plants grown on liquid media, higher levels of the cytokinin nucleotides were detected, probably because of more rapid uptake of dexamethasone directly from the liquid media (Table 1).
Figure 2.
Cytokinin levels in wild-type (wt) and ipt transgenic A. thaliana plants in the 12 h after induction of ipt expression by spraying with 30 μM dexamethasone (Dex). IP, isopentenyladenine; Z, zeatin.
Table 1.
Effect of metyrapone on cytokinin nucleotide content in transgenic Arabidopsis plants when expression of the ipt gene has been induced for 12 h and deuterium labeling of ZMP in induced transgenic plants incubated on D2O-enriched media for 12 h
| Compound | Control | Metyrapone | t test | |
|---|---|---|---|---|
| Cytokinin content, ng⋅g−1 fresh weight | ZMP | 2,200 (61) | 729 (179) | P < 0.01 |
| iPMP | 67 (4) | 42 (2) | P < 0.01 | |
| Deuterium labeling, tracer/tracee ratio | ZMP | 4.5 (0.4) | 2.83 (0.8) | P < 0.05 |
Average with SD in parentheses.
Tracing Cytokinin Biosynthesis by in Vivo Deuterium Labeling.
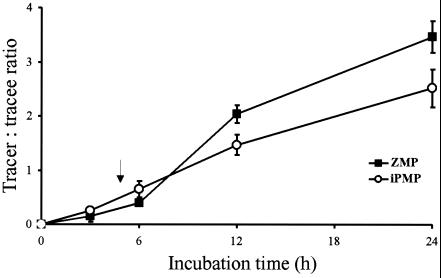
To investigate relative rates of iPMP and ZMP biosynthesis, ipt-transgenic plants were incubated on 2H2O-enriched media containing the inducer. Both cytokinin nucleotides showed significant deuterium enrichments, i.e., increased ratio of labeled to unlabeled cytokinin nucleotides after correction for natural isotope distribution (tracer/tracee ratio) (Fig. 3). The rate of deuterium incorporation into ZMP showed a significant shift (P < 0.05) after approximately 5 h, reflecting the lag time for induction of cytokinin overproduction. Thus, an initial rate of deuterium incorporation, essentially identical to wild-type data, was accelerated by an increased rate of ZMP biosynthesis linked to ipt expression. In contrast, the iPMP pool showed essentially equal rates of deuterium incorporation before and after 5 h of induction, i.e., before and after ipt-mediated cytokinin biosynthesis had been up-regulated. Thus, ipt expression appears to have no significant effect on iPMP biosynthesis rates. Consequently, significantly higher tracer/tracee ratios were found for ZMP than for iPMP after both 12 and 24 h (Fig. 3).
Figure 3.
In vivo deuterium labeling of ZMP (■) and iPMP (○) in transgenic A. thaliana plants, incubated on deuterium-enriched media containing dexamethasone to induce ipt expression. Enrichment is expressed as the ratio of deuterium labeled substance (tracer)/unlabeled substance (tracee), after correction for natural isotope distribution. Initiation of cytokinin overproduction is marked by an arrow. Standard deviation is indicated by error bars. The slope of the ZMP 6- to 12-h regression line was significantly higher than the slope of the 0- to 3-h data (P < 0.05, dummy variable analysis).
In Vivo Deuterium Labeling and Detection of iPMP Conversion.
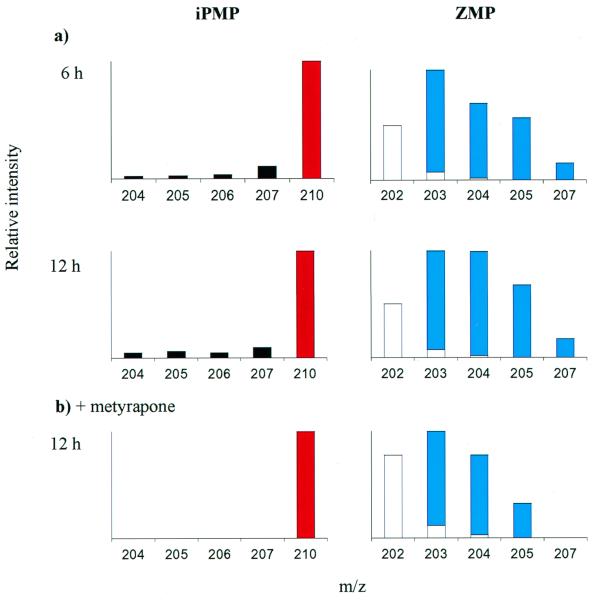
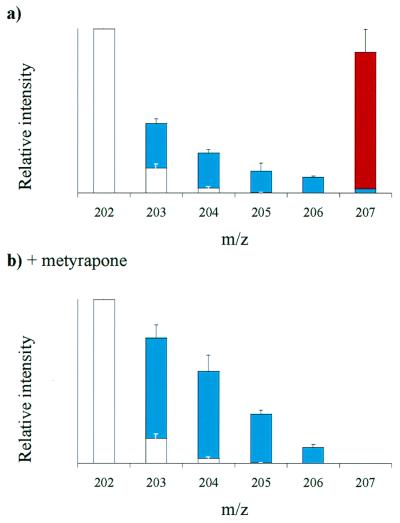
2H2O and [2H6]iPA were fed simultaneously to the ipt-induced transgenic plants, to measure de novo ZMP biosynthesis via in vivo deuterium incorporation, while simultaneously labeling the iPMP pool with [2H6]iPMP, to monitor the postulated conversion of iPMP to ZMP. Allowing [2H6]iPA to be metabolically phosphorylated was the only feasible approach to labeling the iPMP pool, because iPMP is a charged molecule at physiological pH values and does not easily penetrate the plasma membrane, whereas iPA is readily absorbed by plants grown on liquid media and is rapidly metabolized to iPMP (26). The stereo-specific hydroxylation of the [2H6]isopentenyl side chain, labeled at the methyl groups, removes one deuterium, resulting in [2H5]ZMP. Typical profiles from mass isotopomer abundance analysis of iPMP and ZMP after 6 and 12 h of incubation are shown in Fig. 4a. The most abundant iPMP mass isotopomer throughout the experiment was the [2H6]iPMP tracer (m/z 210), and only low deuterium incorporation was observed in the cytokinin base +1 to +4 species (m/z 205–208). In contrast, the ZMP mass isotopomer profiles were dominated by a cluster originating from intense in vivo deuterium labeling, producing abundant +1 to +5 species (m/z 203–207). The [2H5]ZMP species detected in this experiment (6% of the total ZMP pool) could have been produced either by [2H6]iPMP conversion or intense in vivo deuterium labeling of de novo synthesized ZMP. We conclude, however, that de novo production gives rise to most of the ZMP, because the relative abundance of [2H5]ZMP was only 0.2% in induced ipt-transgenic plants incubated on [2H6]iPA-containing media (data not shown).
Figure 4.
(a) Typical mass isotopomer profiles for iPMP and ZMP purified from ipt-expressing A. thaliana plants after feeding with 2H2O/[2H6]iPA double tracers for 6 or 12 h. (b) 12-h double tracer experiment repeated in the presence of metyrapone. Empty bars represent unlabeled ZMP including the natural isotope distribution. Tracers indicated: [2H6]iPMP, red; [2Hn]ZMP species with label originating from 2H2O, blue.
Cytochrome Inhibitor Experiments.
Metyrapone, a cytochrome P450 inhibitor known to inhibit plant isopentenylhydroxylases (15, 27), caused a 67% reduction in ZMP levels in induced ipt-transgenic plants after 12 h (Table 1). It also resulted in a 37% reduction of the iPMP pool, suggesting that it also inhibits cytochrome P450-dependent enzymes upstream of iPMP. Analysis of in vivo deuterium incorporation from deuterated water into ZMP indicated that metyrapone also has an impact on the iPMP-independent biosynthetic pathway (Table 1). Metyrapone also was added during a 2H2O/[2H6]iPA-double tracer experiment. Under these conditions no +5 signal was observed for ZMP in induced ipt-transgenic plants (Fig. 4b), showing that neither de novo biosynthesis nor [2H6]iPMP conversion produced detectable amounts of [2H5]ZMP. The iPMP-dependent synthesis of ZMP was thus completely blocked in this experiment, and the continued presence of a mass isotopomer cluster originated from the deuterated water proved that the plants were able to synthesize ZMP de novo completely independently of the iPMP hydroxylase.
Tracing ZMP Biosynthesis in Wild-Type A. thaliana.
Cytokinin biosynthesis also was examined in wild-type Arabidopsis plants after incubation on 2H2O/[2H6]iPA-double tracer media. The ZMP mass isotopomer profiles from plants incubated for 24 h contained both a mass isotopomer cluster and a discrete +5 signal, the latter demonstrating that the iPMP-dependent pathway was active under these conditions (Fig. 5a). Addition of 2 mM metyrapone blocked the iPMP-dependent pathway by inhibiting the iPMP hydroxylase. The resulting mass isotopomer profile lacked the +5 signal from [2H5]ZMP but contained significant amounts of the lower mass isotopomers from the in vivo ZMP deuterium labeling (Fig. 5b). This finding clearly demonstrates that the wild-type plants were capable of producing ZMP de novo independent of iPMP. With wild-type plants the metyrapone concentration used was lower compared with experiments with ipt-expressing plants to minimize the effect on the iPMP-independent pathway described above.
Figure 5.
(a) ZMP mass isotopomer profiles from A. thaliana wild-type plants incubated in a liquid medium containing 1 μM [2H6]iPA and 30% 2H2O for 24 h. (b) In the presence of metyrapone. Empty bars represent unlabeled ZMP including the natural isotope distribution. Tracers indicated: [2H5]ZMP formed from [2H6]iPMP, red; [2Hn]ZMP species with label originating from 2H2O, blue. Standard deviation is indicated by error bars. Significance level of labeling: P < 0.05 (Student's t test).
Inhibition of Terpenoid Biosynthesis.
The origin of the side-chain precursor used in the alternative pathway was investigated in wild-type plants by using mevastatin, an inhibitor for HMG CoA reductase, a key enzyme in the acetate/mevalonic acid pathway of isoprenoid biosynthesis. In this experiment the iPMP-dependent synthesis was blocked with metyrapone, and addition of mevastatin significantly reduced the in vivo deuterium labeling in ZMP, showing that at least part of the side-chain precursor pool used in the alternative pathway was of terpenoid origin (Fig. 6).
Figure 6.
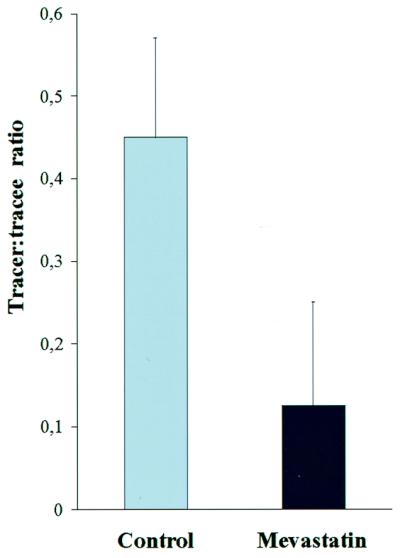
Effect of mevastatin on the in vivo deuterium incorporation into ZMP via the iPMP-independent pathway. Metyrapone added in both experiments to block iPMP-ZMP conversion. Standard deviation is indicated by error bars. P < 0.05 (Student's t test).
Discussion
The primary events of cytokinin biosynthesis generally are thought to be formation of iPMP by conjugation of a 2-isopentenyl hydrocarbon chain to the N6 of AMP (9, 10, 28), followed by rapid, stereo-specific hydroxylation of the side chain to yield ZMP (14, 16, 18). The iPMP-dependent cytokinin biosynthesis pathway (Fig. 7, black arrows) has been extensively studied in tissues expressing the ipt gene from A. tumefaciens, which encodes an enzyme that can catalyze, in vitro, the transfer of an isopentenyl side chain from DMAPP (11, 12) to AMP. Tests of a number of pentenyl compounds as side-chain donors, using partially purified cytokinin-autonomous tobacco callus tissues, demonstrated that substrates with an allylic pyrophosphate group were required for activity (9). However, 4-pyrophosphate-2-methyl-trans-but-2-enol, the most likely candidate for a zeatin type of side-chain donor, has not yet been tested, probably because of the complexity of the required chemical synthesis.
Figure 7.
Biosynthetic scheme describing the iPMP-dependent pathway (black arrows), with iPMP as the primary intermediate and the iPMP-independent pathway (blue arrows) producing ZMP as the first cytokinin. The main flow of the tracers used in this work is indicated: [2H6]iPA, red and 2H2O, blue.
In our study transgenic A. thaliana expressing the ipt gene under the control of a glucocorticoid inducible expression system was used. This system has rapid induction kinetics (21), and ipt transcripts were detected 2–3 h after induction. Surprisingly, ipt expression did not have even a transient impact on the pool size of iPMP, the proposed first product of the isopentenyl transferase (Fig. 2). However, ZMP levels increased 100-fold within 12 h of induction, suggesting either that ZMP was synthesized independently from iPMP, or that iPMP to ZMP conversion remained very rapid even with a very drastic increase of iPMP biosynthesis. ZR levels increased 50-fold, presumably because of the rapid interconversion of nucleotides and nucleosides (26), in accordance with the view that cytokinin biosynthesis occurs mainly at the nucleotide level (28).
We also used a recently developed method where cytokinin biosynthesis was monitored after deuterium incorporation into cytokinins (25). When plants are incubated on a liquid growth medium enriched with deuterium oxide, general labeling occurs throughout essentially all biosynthetic pathways of the plant cell. In pools of particular compounds, unlabeled species are replaced by deuterium-labeled species at rates dependent on the metabolic turnover and the deuterium enrichment of the precursor(s). Deuterium labeling experiments with ipt-transgenic plants indicated that the ZMP biosynthetic rate was significantly higher after ipt expression, but iPMP synthesis essentially remained constant before and after ipt induction (Fig. 3). The difference in biosynthetic rates between ZMP and iPMP can be estimated, assuming that there are two separate side-chain precursors with equal degrees of deuterium enrichment. Using the increase in the tracer/tracee ratio (2) in ZMP (A) and iPMP (B) between 6 and 12 h of incubation (Fig. 3), together with the endogenous pool sizes (Table 1), the difference in biosynthetic rates =
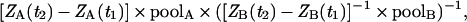 |
1 |
derived from Toffolo et al. (29), the biosynthetic rate of ZMP was calculated to be 66 times higher than that of iPMP.
If deuterium-labeled metabolites are channeled through a linear biosynthetic pathway, the degree of labeling must be higher in early than in late metabolites. In this study, the tracer/tracee ratios of ZMP and iPMP 24 h after induction were 3.5 and 2.5, respectively (Fig. 3). This finding is not consistent with an iPMP-dependent biosynthetic pathway (Fig. 7, black arrows), because a precursor cannot have a lower level of enrichment than its product.
The deduced peptide sequence of the bacterial ipt suggests it has a cytosolic location. Thus, there are only three possible ways to explain our results. First, the ipt also may have hydroxylase activity and produce ZMP directly from DMAPP and AMP in a two-step reaction. However, tests have found no such activity in the ipt (30). Second, the bacterial ipt could form a bienzyme complex with an endogenous plant isopentenylhydroxylase, and substrate channeling could restrict iPMP leakage into the cytosol. However, for efficient metabolite transfer to occur between the two catalytic sites, structural constraints preventing loss of the intermediate into the bulk solution would be essential. The necessary interactions between the associated enzymes in such cases are often highly evolved, as in tryptophan synthase (31), and this would be unexpected between an endogenous and a heterologous enzyme. The third, and likeliest explanation of our findings, is that two different side-chain precursors are used by the ipt in ZMP and iPMP synthesis, in two independent pathways. An alternative, iPMP-independent pathway (Fig. 7, blue arrows) explains not only our data, but also results from earlier studies (13, 18).
The simultaneous use of two tracers, [2H6]iPA and 2H2O, enables analysis of iPMP-hydroxylase activity and de novo biosynthesis in the same experiment. The [2H6]iPA tracer was rapidly converted to [2H6]iPMP and beyond 3 h of incubation, the mass isotopomer profile of iPMP was dominated by the +6 isotopomer from the [2H6] tracer (m/z 210) (Fig. 4). A strong induction of ZMP levels take place in the dexamethasone-ipt plants 6–12 h after induction of ipt expression (Fig. 2). Thus essentially every ZMP molecule was newly synthesized after 12 h of incubation. However, the ZMP profile was not dominated by [2H5]ZMP arising from [2H6]iPMP but by a mass isotopomer cluster produced from in vivo deuterium labeling in a pathway not involving iPMP. Thus, the major precursor of ZMP was not cytoplasmic iPMP.
An objection often raised against feeding experiments is that adding the tracer may uncontrollably alter metabolic processes. Because the total iPA and iPMP pool sizes increase after addition of the [2H6]iPA tracer a potential feedback inhibition of pathways leading into these pools cannot be completely ruled out. A control experiment, however, was performed where [2H5]ZR was fed to increase the pool size of ZMP in a similar manner to the [2H6]iPA feeding. In this experiment the rate of deuterium incorporation into ZMP from deuterated water was measured in wild-type plants, and it was clearly demonstrated that the increased pool sizes of ZMP and ZR did not cause any reduction in iPMP-independent synthesis (data not shown).
Specific inhibitors often are used to investigate metabolic fluxes and metyrapone, a cytochrome P450 inhibitor, has been used to inhibit isopentenylhydroxylases in plants (15), thereby uncoupling the iPMP-independent pathway. However, when induced ipt-transgenic plants were treated with metyrapone, levels of both iPMP and ZMP fell (Table 1). Our observations suggest that metyrapone also inhibits cytokinin metabolism upstream of the nucleotide stages and is not highly specific for the iPMP hydroxylase, in agreement with earlier experiments (27). Metyrapone also significantly reduced the rates of in vivo deuterium incorporation into ZMP (Table 1), indicating a lower rate of biosynthesis in the iPMP-independent pathway. The lower incorporation of deuterium from deuterated water reduced the abundance of higher ZMP as mass isotopomers declined (Fig. 4b). Thus, even small amounts of [2H5]ZMP formed from the [2H6]iPMP tracer would have been detected if the iPMP-hydroxylase had been active. However, no signal was observed in the m/z 207-channel, and therefore it can be concluded that the iPMP hydroxylase was completely inhibited, but with the de novo ZMP biosynthesis maintained because a ZMP mass isotopomer cluster was still present (Fig. 4b). This evidence strongly corroborates the hypothesis that an alternative, iPMP-independent pathway exists in ipt-expressing plants. The experiment also suggests that a cytochrome P450-type enzyme is involved in the synthesis of the side-chain precursor associated with the iPMP-independent pathway.
A further issue addressed was whether the iPMP independent pathway is solely associated with the Agrobacterium ipt, or if it is part of normal cytokinin biosynthesis in plants. Double tracer experiments using Arabidopsis wild-type plants with and without metyrapone clearly demonstrated the presence of an equivalent, iPMP-independent biosynthetic pathway leading to ZMP in wild type. In the controls, both the cluster from in vivo deuterium labeling and a discrete +5 signal from [2H5]ZRMP were observed (Fig. 5a). Addition of metyrapone completely blocked the iPMP conversion, leaving a significant in vivo deuterium incorporation in ZMP (m/z 203–206) (Fig. 5b). Because de novo biosynthesis of ZMP occurred while the iPMP-dependent pathway was inactive, the iPMP-independent pathway also must be involved in endogenous cytokinin biosynthesis in plants.
Finally, the terpenoid origin of the Z-type of side-chain precursor was tested by adding mevastatin to an in vivo deuterium labeling experiment in wild-type plants where the iPMP–ZMP conversion was blocked with metyrapone. Mevastatin inhibits HMG CoA reductase and thereby blocks the cytosolic acetate/mevalonic acid pathway of terpenoid biosynthesis, with reduced cytosolic levels of DMAPP as a consequence. Application of HMG CoA inhibitors has been shown to reduce the levels of zeatin-type cytokinins (32), which might, at least in part, be mediated by the iPMP-independent cytokinin biosynthesis pathway. In the present investigation, significant reduction of deuterium incorporation into ZMP, in the presence of both metyrapone and mevastatin, indicates that the iPMP-independent pathway uses a side-chain donor of terpenoid origin (Fig. 6). The result also indicates that the endogenous iPMP-independent pathway has a cytosolic localization because isoprenoid biosynthesis in plastids is independent of mevalonate. In the chloroplast, DMAPP and isopentenyl-pyrophospate (IPP) are formed by the 1-deoxy-d-xylulose-5-phosphate pathway, starting with glyceraldehyde-3-phosphate and pyruvate (33), and only a limited cross talk between the two DMAPP/IPP pathways has been indicated by labeling experiments (34).
We have now provided evidence for the existence of an iPMP-independent cytokinin biosynthesis pathway, both in A. thaliana expressing the A. tumefaciens ipt gene and as part of normal wild-type plant metabolism. Although in the transgenic tissues the majority of the synthesis is mediated via the iPMP-independent pathway, both pathways seem to be important in wild-type tissue. The relative contribution of the two pathways may vary in a tissue- and time-dependent manner. Our data also indicate that the Z-type side-chain precursor is synthesized as a branch-off from the terpenoid pathway. This, and other aspects of cytokinin biosynthesis, could be probed in further studies using the labeling technology applied here.
Acknowledgments
We thank Roger Granbom for skillful technical assistance. This work was supported by grants from the Swedish Natural Sciences Research Council (NFR) and the Swedish Council of Forestry and Agricultural Research (SJFR). Work at the Rockefeller University was supported by National Institutes of Health Grant GM44640 (to N.-H.C.). T.K. was supported by a Human Frontier Science Progam postdoctoral fellowship.
Abbreviations
- DMAPP
dimethylallyl-pyrophosphate
- ZR
zeatinriboside
- ZMP
zeatinriboside-5′-monophospate
- iPA
isopentenyladenosine
- iPMP
isopentenyladenosine-5′-monophosphate
- ipt
isopentenyltransferase
- HMG
3-hydroxy-3-methylglutaryl
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260504097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260504097
References
- 1.Miller C O, Skoog F, Von Saltza M H, Strong F M. J Am Chem Soc. 1955;78:1392. [Google Scholar]
- 2.Skoog F, Strong F M, Miller C O. Science. 1965;148:532–533. doi: 10.1126/science.148.3669.532-a. [DOI] [PubMed] [Google Scholar]
- 3.Mok M C. In: Cytokinins: Chemistry, Activity and Function. Mok D W S, Mok M C, editors. London: CRC; 1994. pp. 155–166. [Google Scholar]
- 4.Swaminathan S, Bock R M. Biochemistry. 1977;16:1355–1360. doi: 10.1021/bi00626a018. [DOI] [PubMed] [Google Scholar]
- 5.Klämbt D. In: Physiology and Biochemistry of Cytokinins in Plants. Kaminek M, Mok D W S, Zazímalová E, editors. The Hague: SPB Academic Publishing; 1992. pp. 25–27. [Google Scholar]
- 6.Taya Y, Tanaka Y, Nishimura S. Nature (London) 1978;271:545–547. doi: 10.1038/271545a0. [DOI] [PubMed] [Google Scholar]
- 7.Miura G A, Miller C O. Plant Physiol. 1969;44:372–376. doi: 10.1104/pp.44.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura G, Hall R H. Plant Physiol. 1973;51:563–569. doi: 10.1104/pp.51.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C M. In: Plant Growth Substances 1982. Wareing P F, editor. London: Academic; 1982. pp. 155–163. [Google Scholar]
- 10.Letham D S, Palni L M S. Annu Rev Plant Phys. 1983;34:163–197. [Google Scholar]
- 11.Barry G F, Rogers S G, Fraley R T, Brand L. Proc Natl Acad Sci USA. 1984;81:4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyoshi D E, Klee H, Amasino R M, Nester E W, Gordon M P. Proc Natl Acad Sci USA. 1984;81:5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuchbury T, Palni L M, Horgan R, Wareing P F. Planta. 1979;147:97–102. doi: 10.1007/BF00389507. [DOI] [PubMed] [Google Scholar]
- 14.Palni L M S, Horgan R. Phytochemistry. 1983;22:1597–1601. [Google Scholar]
- 15.Chen C-M, Leisner S M. Plant Physiol. 1984;75:442–446. doi: 10.1104/pp.75.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redig P, Schmulling T, Van Onckelen H. Plant Physiol. 1996;112:141–148. doi: 10.1104/pp.112.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eklöf S, Åstot C, Moritz T, Blackwell J, Olsson O, Sandberg G. Physiol Plant. 1996;98:333–334. [Google Scholar]
- 18.Zhang R, Zhang X, Wang J, Letham D S, McKinney S A, Higgins T J V. Planta. 1995;196:84–94. [Google Scholar]
- 19.Hare P D, van Staden J. Physiol Plant. 1994;91:128–136. [Google Scholar]
- 20.Motyka V, Faiss M, Strnad M, Kaminek M, Schmulling T. Plant Physiol. 1996;112:1035–1043. doi: 10.1104/pp.112.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama T, Chua N H. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- 22.Kunkel T, Niu Q W, Chan Y S, Chua N H. Nat Biotechnol. 1999;17:916–919. doi: 10.1038/12914. [DOI] [PubMed] [Google Scholar]
- 23.Kang H G, Fang Y, Singh K B. Plant J. 1999;20:127–133. doi: 10.1046/j.1365-313x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 24.Åstot C, Dolezal K, Moritz T, Sandberg G. J Mass Spectrom. 1998;33:892–902. doi: 10.1002/(SICI)1096-9888(199809)33:9<892::AID-JMS701>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Åstot C, Dolezal K, Moritz T, Sandberg G. J Mass Spectrom. 2000;35:13–22. doi: 10.1002/(SICI)1096-9888(200001)35:1<13::AID-JMS901>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Jameson P E. In: Cytokinins: Chemistry, Activity, and Function. Mok D W S, Mok M C, editors. London: CRC; 1994. pp. 113–124. [Google Scholar]
- 27.Long A R, Chism G W. Biochem Biophys Res Commun. 1987;144:109–144. doi: 10.1016/s0006-291x(87)80482-5. [DOI] [PubMed] [Google Scholar]
- 28.Chen C-M. Physiol Plant. 1997;101:665–673. [Google Scholar]
- 29.Toffolo G, Foster D M, Cobelli C. Am J Physiol. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- 30.Heinemeyer W, Buchmann I, Tonge D W, Windass J D, Altmoerbe J, Weiler E W, Botz T, Schroder J. Mol Gen Genet. 1987;210:156–164. [Google Scholar]
- 31.Pan P, Woehl E, Dunn M F. Trends Biochem Sci. 1997;22:22–27. doi: 10.1016/s0968-0004(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 32.Laureys F, Dewitte W, Witters E, Van Montagu M, Inze D, Van Onckelen H. FEBS Lett. 1998;426:29–33. doi: 10.1016/s0014-5793(98)00297-x. [DOI] [PubMed] [Google Scholar]
- 33.Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtenthaler H K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–50. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]