Abstract
Background
Listeria monocytogenes has been implicated in several food borne outbreaks as well as sporadic cases of disease. Increased understanding of the biology of this organism is important in the prevention of food borne listeriosis.
The infectivity of Listeria monocytogenes ScottA, cultivated with and without oxygen restriction, was compared in vitro and in vivo. Fluorescent protein labels were applied to allow certain identification of Listeria cells from untagged bacteria in in vivo samples, and to distinguish between cells grown under different conditions in mixed infection experiments.
Results
Infection of Caco-2 cells revealed that Listeria cultivated under oxygen-restricted conditions were approximately 100 fold more invasive than similar cultures grown without oxygen restriction. This was observed for exponentially growing bacteria, as well as for stationary-phase cultures.
Oral dosage of guinea pigs with Listeria resulted in a significantly higher prevalence (p < 0.05) of these bacteria in jejunum, liver and spleen four and seven days after challenge, when the bacterial cultures had been grown under oxygen-restricted conditions prior to dosage. Additionally, a 10–100 fold higher concentration of Listeria in fecal samples was observed after dosage with oxygen-restricted bacteria. These differences were seen after challenge with single Listeria cultures, as well as with a mixture of two cultures grown with and without oxygen restriction.
Conclusion
Our results show for the first time that the environmental conditions to which L. monocytogenes is exposed prior to ingestion are decisive for its in vivo infective potential in the gastrointestinal tract after passage of the gastric barrier. This is highly relevant for safety assessment of this organism in food.
Background
During the last two decades, Listeria monocytogenes has been implicated in several food borne outbreaks and sporadic cases of disease [1,2]. Foods implicated in foodborne listeriosis are generally highly processed foods with prolonged shelflives, supportive of growth of the organism [3]. Several attempts have been made to establish quantitative microbiological criteria for the presence of L. monocytogenes in foods [4,5], by use of dose-response predictions. However, there are indications that the number of bacteria ingested is not the only important determinant for the development of illness. For many enteric pathogens also including Listeria, it is well known that given environmental conditions induce the expression of identified virulence genes and/or contribute to their invasive potential in in vitro models [6-15]. However, to our knowledge, no reports describe a direct effect of such conditions on the virulence phenotype of these bacteria in vivo. It has thus not been shown whether an induced expression of virulence factors in intestinal pathogens at the time of ingestion affects their virulence in vivo after passage of the gastric barrier.
The objective of the present study was to investigate whether the physiological state of L. monocytogenes prior to ingestion (i.e. determined by the food environment), here exemplified by oxygen availability, could influence its ability to cause infection.
Recent reports point out that mice and rats are not suitable as animal models for human listerial infectivity, since the bacterium does not interact with the epithelial receptor of these animals [16,17]. A guinea pig model [BB Roldgaard, JB Andersen, TR Licht and BB Christensen, submitted] was therefore used for in vivo studies of infectivity in the present study, while Caco-2 cells were applied to assess the infective potential in vitro. In any animal model, variation between individuals is unavoidable. In order to circumvent this variation in the comparative study, challenge of the animals with a mixture of L. monocytogenes ScottA cells cultivated under conditions of different oxygen availability, was included in the investigation. For this purpose, a recently developed fluorescence labeling system [18] was applied, making it possible to distinguish between otherwise isogenic Listeria cells originating from cultures grown with and without oxygen-restriction.
The obtained results reveal that oxygen-restriction clearly increases the infective potential of L. monocytogenes in vitro and in vivo.
Results
Invasiveness of oxygen-restricted and un-restricted L. monocytogenes in Caco-2 cells
Caco-2 cell assays revealed that cultures of L. monocytogenes-CFP, grown to selected densities under oxygen-restricted conditions were approximately 100 fold more invasive than corresponding cultures grown without oxygen restriction (Figure 1). Similar results were obtained with L. monocytogenes-YFP, as well as with stationary-phase overnight cultures grown with and without oxygen restriction (data not shown).
Figure 1.
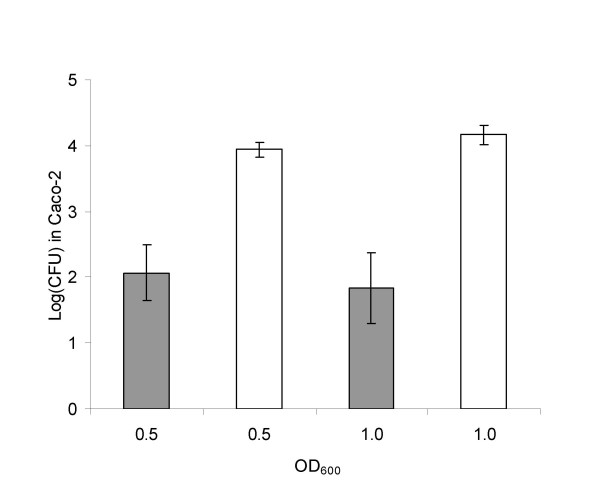
In vitro infectivity. Numbers of invaded un-restricted (grey) and oxygen-restricted (white) L. monocytogenes-CFP per well of Caco-2 cells grown to selected densities. Counts were normalized to a concentration of 107 L. monocytogenes per ml in the bacterial cultures prior to infection. Each bar represents an average of three different experiments. Error bars designate standard deviations. OD600 refers to optical density at 600 nm.
Infection of guinea pigs with monocultures of oxygen- restricted and un-restricted L. monocytogenes
Exponentially growing cultures (OD600 = 1.0) were used for oral dosage of guinea pigs. L. monocytogenes-CFP was recovered from the liver and jejunum of half of the 12 animals dosed twice with the un-restricted bacterial cultures, and was found in the spleen of a single animal. The occurrence of Listeria in organs of animals dosed with oxygen-restricted bacteria was significantly higher (p < 0.05); the pathogen was recovered from jejunum of all of the 12 animals, and was found in the liver and spleen of ten and seven of the guinea pigs, respectively. The average concentrations of Listeria found in positively infected organs were not different in the two groups (Table 1).
Table 1.
L. monocytogenes monoculture infections in guinea pigs
| Un-restricted | Oxygen-restricted | |||||||
| Days post first dosage | 4 | 7 | Total | Mean log(CFU/g) | 4 | 7 | Total | Mean log(CFU/g) |
| Liver | 3/6 | 3/6 | 6/12 (50%) | 2.2 ± 0.34 | 6/6 | 4/6 | 10/12 (83%) | 3.0 ± 0.88 |
| Spleen | 0/6 | 1/6 | 1/12 (8%) | 2.8 | 4/6 | 3/6 | 7/12 (58%) | 2.6 ± 0.67 |
| Jejunum | 2/6 | 4/6 | 6/12 (50%) | 2.1 ± 0.47 | 6/6 | 6/6 | 12/12 (100%) | 3.7 ± 0.70 |
Numbers of guinea pigs where L. monocytogenes-CFP was recovered from liver and spleen after oral dosage with un-restricted or oxygen-restricted L. monocytogenes-CFP, respectively. Mean log (CFU/g) designate the mean levels found in animals positive for Listeria in the given organs, followed by standard deviations.
Throughout the experiment, fecal counts of Listeria remained significantly higher in the animals dosed with oxygen-restricted L. monocytogenes-CFP than in those dosed with the un-restricted, identical strain. The concentration of Listeria in feces reached a level of 106–107 per gram on the days immediately after dosage, which was carried out on Day 0 and Day 1 of the experiment. Hereafter, the concentration in animals dosed with L. monocytogenes grown under un-restricted conditions dropped and remained at a level of approximately 103, while the fecal Listeria concentration in animals dosed with oxygen-restricted cells stayed between 1 and 3 logs higher (Figure 2A).
Figure 2.
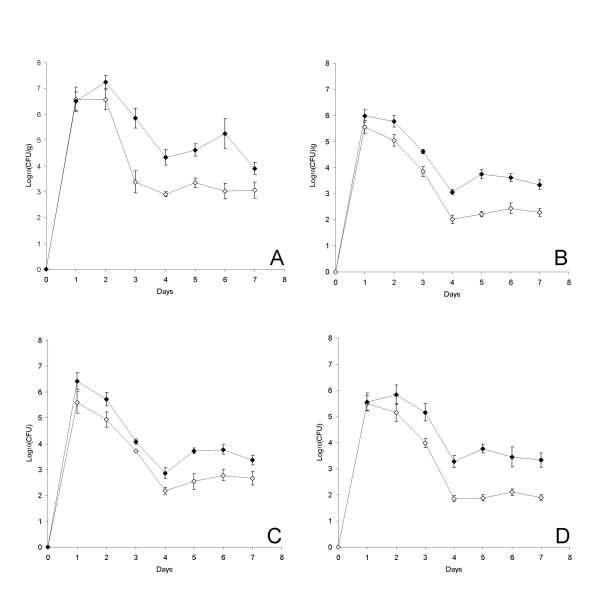
Fecal densities. Concentration of L. monocytogenes in fecal samples of animals dosed with un-restricted (open symbols) and/or oxygen-restricted (closed symbols), fluorescence-labeled bacteria. (A): Animals dosed with monocultures of CFP-labeled bacteria. (B): Integrated presentation of data from C and D. (C): Animals dosed with a mixture of CFP-labeled, un-restricted Listeria and YFP-labeled, oxygen-restricted Listeria. (D): Animals were dosed with a mixture of YFP-labeled, un-restricted Listeria and CFP-labeled, oxygen-restricted Listeria. All animals were dosed at Day 0 and again at Day 1. Each data point in panel A and B represents the average of samples from 12 animals, while each data point in panel C and D represents the average of samples from 6 animals. Error bars designate standard errors of the means.
Competitive infection of guinea pigs with oxygen-restricted and un-restricted L. monocytogenes
Oral dosage of 24 guinea-pigs with a mixture of oxygen-restricted and un-restricted, exponentially growing L. monocytogenes revealed that also in a mixed challenge experiment, the occurrence of the oxygen-restricted strain in internal organs was significantly higher (p < 0.05) than observed for the un-restricted strain. Listeria, which had been grown under un-restricted conditions, was detected in the liver and jejunum of 2 animals, and in the spleen of 4 animals, while Listeria grown under oxygen-restricted conditions prior to dosage was recovered from liver, spleen and jejunum of 18, 12, and 14 animals, respectively. It had no influence on the infectivity (p = 0.165) whether the L. monocytogenes cells were labeled with CFP or YFP. The average concentrations of Listeria found in positively infected organs were similar for oxygen-restricted and un-restricted bacteria (Table 2).
Table 2.
L. monocytogenes mixed culture infections in guinea pigs
| Un-restricted | Oxygen-restricted | |||||||
| Fluorescence | CFP | YFP | Total | Mean log(CFU/g) | CFP | YFP | Total | Mean log(CFU/g) |
| Liver | 1/12 | 1/12 | 2/24 (8%) | 1.5 ± 0.21 | 10/12 | 8/12 | 18/24 (75%) | 1.9 ± 0.32 |
| Spleen | 3/12 | 1/12 | 4/24 (17%) | 1.9 ± 0.76 | 7/12 | 5/12 | 12/24 (50%) | 2.1 ± 0.58 |
| Jejunum | 1/12 | 1/12 | 2/24 (8%) | 2.2 ± 0.21 | 8/12 | 6/12 | 14/24 (58%) | 3.1 ± 0.65 |
Numbers of guinea pigs where L. monocytogenes was recovered from liver and spleen after oral dosage with a 1:1 mixture of un-restricted and oxygen-restricted L. monocytogenes, carrying either of the two different fluorescent labels CFP and YFP. Samples were taken either four or seven days post first dosage. Mean log (CFU/g) designate the mean levels found in animals positive for Listeria in the given organs, followed by standard deviations.
In all animals, fecal concentrations of Listeria grown under oxygen-restricted conditions prior to dosage were higher than concentrations of cells grown without oxygen restriction (Figure 2B). This was observed independently of which fluorescent label was used to identify the bacteria (Figure 2C and 2D). The kinetics of the pathogen occurrence in feces observed in the mixed infection experiment were quite similar to what was observed after dosage with monocultures (Figure 2).
Discussion
We have shown that oxygen-restriction prior to ingestion significantly (p < 0.05) enhances the in vivo infective potential of L. monocytogenes ScottA (Table 1 and 2, Figure 2). This has to our knowledge not previously been shown, and signifies that gene expression occurring in Listeria before intake influences its infective potential even after passage of the oral/gastric barrier. The trend in the results is that the prevalence of Listeria in internal organs increases from Day 4 to Day 7 post challenge with the un-restricted bacteria, while a decrease in prevalence is seen in the same period after dosage with the oxygen-restricted bacteria (Table 1). The effect of oxygen-restriction prior to dosage is thus larger on Day 4 than on Day 7 post dosage, which is not surprising because the expression patterns present in the cultures at the time of ingestion must be expected to approach each other over time, since the cells are all exposed to similar environmental conditions in the gut.
While oxygen restriction clearly affected the number of animals carrying L. monocytogenes in their internal organs, the concentration of bacteria present in positively infected organs was not affected (Table 1 and 2). This suggest that oxygen restriction increases the initial translocation of Listeria from the gut lumen to internal organs, but does not influence the ability of the bacteria to proliferate inside the investigated organs.
Obviously, an increased ability to survive the gastric barrier will increase the probability of causing an infection [19,20]. It is well known, that genes involved in many kinds of stress-responses are co-regulated, and that exposure to one type of stress therefore improves the ability to survive another type of stressful condition [21,22]. It could thus be speculated, that exposure to oxygen restriction would increase the viability of L. monocytogenes under low pH and/or its resistance to gastric enzymes. This is however not the full explanation for our observations, since also the ability of this strain to infect Caco-2 cells in vitro is significantly increased by oxygen restriction (Figure 1). In the in vitro studies, the effect of oxygen restriction was seen for exponentially growing cultures as well as for cultures in stationary phase, and the induction of the virulent phenotype was thus expected to be independent of the growth stage of the bacterial cells (Figure 1). Still, we chose to use exponentially growing cultures for the animal studies in order to eliminate putative contributions to in vivo infectivity from the many genes known to be induced in stationary phase.
We suggest that the observed increased infectivity of L. monocytogenes grown under oxygen-restricted conditions can be attributed to an increased expression of the InternalinA (InlA) protein, which is known to be a key factor for virulence of L. monocytogenes [23], on the surface of the bacterial cells. In concordance with this hypothesis, recent reports indicate that anaerobic physiology contributes significantly to an enhanced production of InlA in aro mutants of L. monocytogenes [24,25]. InlA mediates the interaction of Listeria cells with specific receptors (E-cadherin) in the human gut [26], and may therefore be important for bacterial attachment to the epithelial wall. We observed that oxygen-restriction significantly increased the prevalence of L. monocytogenes in the jejunum of guinea-pigs (Table 1 and 2), suggesting that an increased InlA-expression occurring prior to ingestion caused increased attachment of L. monocytogenes to the jejunal mucosa. We speculate that the observed increased translocation to spleen and liver (Tables 1 and 2), as well as the increased invasion of Caco-2 cells (Figure 1) may be attributed to an increased initial InlA-mediated attachment to the epithelial receptors. However, also other genes are reported to be induced under oxygen restricted conditions and to affect attachment of Listeria [27].
The percentage of animals in which Listeria was recovered from internal organs was significantly lower (p < 0.05) in animals infected with mixed cultures, than in animals infected with monocultures (Tables 1 and 2). The sensitivity of the mixed culture infection approach was thus slightly lower than observed for infection with monocultures, probably because the dosed numbers of cells grown under a given condition in the mixed infections were only half of the corresponding numbers dosed as monocultures. Total numbers of cells in each dosage were similar in the two approaches. In spite of this, the observed difference between oxygen-restricted and un-restricted Listeria was more significant in the mixed culture experiment (pliver < 0.0001; pspleen = 0.0143; pjejunum = 0.0002) than in the monoculture infections (pliver = 0.0833; pspleen = 0.0094; pjejunum = 0.0047) due to the higher amount of data resulting from the mixed-infection approach. This shows that the co-infection model, which has the advantage of elimination of variations attributed to individual animals, additionally had a better discriminatory power than a monoculture approach involving the same number of animals.
Conclusion
Our results are of particular importance for the risk assessment of Listeria in food. For the first time, we have shown that the environmental conditions to which a bacterium is exposed before ingestion can be decisive for its infective potential when it reaches the gut. This means that not only the number of Listeria present in a given food item, but that also the physiological condition of these bacteria is important for food safety. The in vitro and in vivo data suggest that an oxygen-restricted L. monocytogenes cell represents a significantly higher risk than a cell grown without oxygen restriction. This should be taken into account in future quantitative risk profiling and dose-response models.
Methods
Strains and media
The clinical isolate, Listeria monocytogenes ScottA, carrying erythromycin resistance, and labeled with either Cyan Fluorescent Protein (CFP) or Yellow Fluorescent Protein (YFP) as previously described [18] was used in all experiments. Bacteria were cultivated on BHI-agar (Oxoid) or in liquid BHI (Oxoid) buffered with 100 mM 3-(N-Morpholino)-propanesulfonic acid (MOPS), pH = 6. When appropriate, Erythromycin (Sigma) was used at a final concentration of 10 ug/mL and Nalidixic acid at a final concentration of 100 ug/mL.
Preparation of L. monocytogenes for infection studies
A fluorescent single colony of L. monocytogenes was inoculated into 10 mL MOPS buffered BHI media supplemented with Erythromycin and incubated at 37°C for 8 hours. The resulting cultures, for which the optical densies (OD600) ranged between 1,5 and 2, were subsequently diluted between 104 and 105 fold into 2 L Bluecap flasks containing 450 mL MOPS buffered BHI medium supplemented with Erythromycin.
To obtain oxygen-restricted cultures of Listeria monocytogenes ScottA, atmospheric air above the diluted cultures were exchanged with sterile Nitrogen and the lid of the Bluecap flasks was tightened and sealed prior to incubation. To obtain non-restricted cultures, the Bluecap flasks were incubated with the lid loosely tightened allowing free exchange of atmospheric air. All cultures were incubated at 37°C in a rotary shaker set at 200 rpm and samples for in vitro invasion studies and in vivo infection studies were taken after approximately 20 hours of incubation, when they reached the optical densities reported below.
Samples for in vitro invasion studies were taken at OD600 = 0.5 (exponential phase), OD600 = 1.0 (exponential phase), and from cultures that had been in stationary phase for approximately 10 hours (referred to as over night cultures). The samples were diluted directly into 37°C MEM medium (Gibco) to a concentration of 107 bacteria/mL and immediately used for challenge of Caco-2 cells as described below.
Samples for oral challenge of guinea pigs were cultivated until OD600 = 1, and 400 mL culture per group of 6 animals was harvested by centrifugation (5 minutes, 7000 g, 25°C). The pellet was resuspended in 4 mL of double cream (38 % fat, pH 7). Aliqouts of 0.5 mL, containing either Listeria monocultures, or a mixture of two cultures grown at oxygen restricted and unrestricted conditions, respectively, were subsequently administered to guinea pigs as described below. Samples of the inoculums were diluted and spread onto BHI-agar supplemented with Erythromycin to estimate the numbers of Listeria present in the double cream suspensions. Microscopy revealed that the bacteria were present in the hydric phase.
Caco-2 cell infection experiments
Enterocyte-like Caco-2 cells were cultivated and prepared as previously described [28]. Bacteria were cultivated as described above to the desired optical density and diluted in 37°C MEM medium (Gibco) to a concentration of 107 bacteria/mL immediately before the invasion assays. One ml of bacterial culture was applied to each well, resulting in a multiplicity of infection of approximately 25 bacteria per Caco-2 cell. Following 1 hour of invasion and 2 hours of gentamycin treatment to kill extracellularly located bacteria, Caco-2 cells were lysed and the numbers of Listeria present in each well was estimated as described in the section 'Enumeration of L. monocytogenes in samples'. Sampling was done in triplicate, and the experiments were performed twice.
Animal experiments
Male and female Hartley guinea pigs (Charles River Laboratories; Germany) with a weight of 275 g (± 10 g) were used. After seven days of acclimatization in pens (custom made, 90 × 130 × 61 cm), animals were randomized and housed individually in Polycarbonate cages, Eurostandard Type III H (425 × 266 × 185 mm) with Tapvei bedding (peeled Aspen hardwood, Tapvei Kaavi, Finland) in negatively pressurized isolators. Fecal samples from the guinea pigs were tested by plating on Palcam agar (Oxoid) to verify the absence of Listeria prior to dosage.
A total of 48 guinea pigs were dosed with L. monocytogenes ScottA. Two groups of 12 animals were dosed with monocultures of CFP-labelled bacteria, cultivated either with or without oxygen-restriction. Two other groups of 12 animals were dosed with a 1:1 mixture of oxygen-restricted and un-restricted bacteria carrying the two different fluorescent labels CFP and YFP. In one of these groups, it were the oxygen-restricted Listeria, which carried the CFP label, while in the other group the labels were reversed so that the unrestricted bacteria were labelled with CFP.
All animals were dosed with 0.5 ml double cream containing 38 % milk fat and approximately 5 × 1010 L. monocytogenes at Day 0 and again at Day 1. The number of cells in the inoculum was approximately the same in the mono- and mixed cultures.
Dosage was done directly in the oral cavity, between the incisors and the molars. Following dosage the animals had access to food and water ad libitum throughout the duration of the study. Fresh fecal samples were collected every day, avoiding bedding and traces of urin from the cages. Six animals from each of the groups were euthanized on Days 4 and 7, respectively, for investigation of intestinal segments, liver and spleen. Samples of jejunal content from the middle part of the small intestine were squeezed out with tweezers, and samples from spleen and liver of approximately 0.6 g were homogenized prior to investigation as described below.
Ethical aspects
Animal experiments were carried out under the supervision of the Danish National Agency for Protection of Experimental Animals
The recently developed guinea-pig model [BB Roldgaard, JB Andersen, TR Licht and BB Christensen, submitted] is much less stressful to the animals than previously published models [17], since it involves no intubation, injection or anesthetizing. Additionally, the approach of treating each animal with a mixture of cultures reduces the number animals needed.
Estimation of L. monocytogenes in samples
Samples of lysed Caco-2 cells and homogenized organs were cultivated on BHI-agar supplemented with Erythromycin. Samples from faeces and jejunum were cultivated on BHI-agar supplemented with Erythromycin and Nalidixic acid. After 48–72 hours of incubation at 37°C, BHI plates were placed on a UV table (excitation at 302 nm), and fluorescent colonies (either CFP or YFP) were enumerated.
Statistics
Statistical analysis was performed using the software package JMP (SAS institute, Copenhagen, Denmark). Pearsons Chi2 test was used to compare the numbers of infected animals after dosage with either oxygen-restricted or un-restricted L. monocytogenes. The same test was used to compare the results obtained with either mono infection or mixed infection, and with either YFP or CFP labelling. A significance level of 0.05 was used in all cases.
Authors' contributions
JBA carried out the sample preparation and the in vitro invasion assays, while BBR was responsible for the animal experiments. BBC and TRL conceived the study and participated in its design and coordination. TRL drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Grethe Fisher and Mansour Badaki for excellent technical assistance, and Tina Beck Hansen for help with statistical analysis. Anne Ørngreen and her staff are acknowledged for assistance with animal handling. The Danish Councils for Independent Research and The Danish Council for Strategic Research supported this work.
Contributor Information
Jens Bo Andersen, Email: jeban@food.dtu.dk.
Bent B Roldgaard, Email: bebro@food.dtu.dk.
Bjarke Bak Christensen, Email: bbc@food.dtu.dk.
Tine Rask Licht, Email: trl@food.dtu.dk.
References
- Low JC, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J. 1997;153:9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Authority EFS. Trends and Sources of zoonoses, zoonotic agents and antimicrobial resistance in the European Union in 2004. The EFSA Journal 2005 - 310. 2006.
- Foundation ILSIR, Institute RS. Achieving continuous improvement in reductions in foodborne listeriosis--a risk-based approach. J Food Prot. 2005;68:1932–1994. doi: 10.4315/0362-028x-68.9.1932. [DOI] [PubMed] [Google Scholar]
- Rocourt J, BenEmbarek P, Toyofuku H, Schlundt J. Quantitative risk assessment of Listeria monocytogenes in ready-to-eat foods: the FAO/WHO approach. FEMS Immunol Med Microbiol. 2003;35:263–267. doi: 10.1016/S0928-8244(02)00468-6. [DOI] [PubMed] [Google Scholar]
- Norrung B. Microbiological criteria for Listeria monocytogenes in foods under special consideration of risk assessment approaches. Int J Food Microbiol. 2000;62:217–221. doi: 10.1016/s0168-1605(00)00338-x. [DOI] [PubMed] [Google Scholar]
- Kim H, Marquis H, Boor KJ. SigmaB contributes to Listeria monocytogenes invasion by controlling expression of inlA and inlB. Microbiology. 2005;151:3215–3222. doi: 10.1099/mic.0.28070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Boor KJ, Marquis H. Listeria monocytogenes {sigma}B Contributes to Invasion of Human Intestinal Epithelial Cells. Infect Immun. 2004;72:7374–7378. doi: 10.1128/IAI.72.12.7374-7378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sue D, Fink D, Wiedmann M, Boor KJ. {sigma}B-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi: 10.1099/mic.0.27257-0. [DOI] [PubMed] [Google Scholar]
- Lee CA, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst RK, Dombroski DM, Merrick JM. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos JJ. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturongakul S, Boor KJ. SigmaB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl Environ Microbiol. 2006;72:5197–5203. doi: 10.1128/AEM.03058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins JN, Mottaz HM, Norbeck AD, Gustin JK, Rue J, Clauss TR, Purvine SO, Rodland KD, Heffron F, Smith RD. Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol Cell Proteomics. 2006;5:1450–1461. doi: 10.1074/mcp.M600139-MCP200. [DOI] [PubMed] [Google Scholar]
- Rychlik I, Barrow PA. Salmonella stress management and its relevance to behaviour during intestinal colonisation and infection. FEMS Microbiol Rev. 2005;29:1021–1040. doi: 10.1016/j.femsre.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Chaturongakul S, Boor KJ. RsbT and RsbV Contribute to {sigma}B-Dependent Survival under Environmental, Energy, and Intracellular Stress Conditions in Listeria monocytogenes. Appl Environ Microbiol. 2004;70:5349–5356. doi: 10.1128/AEM.70.9.5349-5356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, Babinet C, Cossart P. A Transgenic Model for Listeriosis: Role of Internalin in Crossing the Intestinal Barrier. Science. 2001;292:1722–1725. doi: 10.1126/science.1059852. http://www.sciencemag.org/cgi/content/abstract/292/5522/1722 [DOI] [PubMed] [Google Scholar]
- Andersen JB, Roldgaard BB, Lindner AB, Christensen BB, Licht TR. Construction of a multiple fluorescence labelling system for use in co-invasion studies of Listeria monocytogenes. BMC Microbiology. 2006;6:86. doi: 10.1186/1471-2180-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audia JP, Webb CC, Foster JW. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int J Med Microbiol. 2001;291:97–106. doi: 10.1078/1438-4221-00106. [DOI] [PubMed] [Google Scholar]
- Richard HT, Foster JW. Acid resistance in Escherichia coli. Adv Appl Microbiol. 2003;52:167–186. doi: 10.1016/s0065-2164(03)01007-4. [DOI] [PubMed] [Google Scholar]
- Renzone G, D'Ambrosio C, Arena S, Rullo R, Ledda L, Ferrara L, Scaloni A. Differential proteomic analysis in the study of prokaryotes stress resistance. Ann Ist Super Sanita. 2005;41:459–468. [PubMed] [Google Scholar]
- O'Driscoll B, Gahan CG, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner MR, Njaa BL, Wiedmann M, Boor KJ. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect Immun. 2006;74:876–886. doi: 10.1128/IAI.74.2.876-886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritzker J, Janda J, Schoen C, Taupp M, Pilgrim S, Gentschev I, Schreier P, Geginat G, Goebel W. Growth, Virulence, and Immunogenicity of Listeria monocytogenes aro Mutants. Infect Immun. 2004;72:5622–5629. doi: 10.1128/IAI.72.10.5622-5629.2004. http://iai.asm.org/cgi/content/abstract/72/10/5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritzker J, Schoen C, Goebel W. Enhanced Synthesis of Internalin A in aro Mutants of Listeria monocytogenes Indicates Posttranscriptional Control of the inlAB mRNA. J Bacteriol. 2005;187:2836–2845. doi: 10.1128/JB.187.8.2836-2845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, Gordon JI, Cossart P. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: Role of internalin interaction with trophoblast E-cadherin. PNAS. 2004;101:6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary PL, Allen RD, Dons L, Kathariou S. Insertional inactivation of the Listeria monocytogenes cheYA operon abolishes response to oxygen gradients and reduces the number of flagella. Can J Microbiol. 1999;45:646–652. [PubMed] [Google Scholar]
- Larsen CN, Norrung B, Sommer HM, Jakobsen M. In Vitro and In Vivo Invasiveness of Different Pulsed-Field Gel Electrophoresis Types of Listeria monocytogenes. Appl Environ Microbiol. 2002;68:5698–5703. doi: 10.1128/AEM.68.11.5698-5703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


