Abstract
The omega-3 fatty acids (FAs) found in fish and fish oils (eicosapentaenoic and docosahexaenoic acids, EPA and DHA) have been reported to have a variety of beneficial effects in cardiovascular diseases. Ecological and prospective cohort studies as well as randomized, controlled trials have supported the view that the effects of these FAs are clinically-relevant. They operate via several mechanisms, all beginning with the incorporation of EPA and DHA into cell membranes. From here, these omega-3 FA alter membrane physical characteristics and the activity of membrane-bound proteins, and once released by intracellular phospholipases, can interact with ion channels, be converted into a wide variety of bioactive eicosanoids, and serve as ligands for several nuclear transcription factors thereby altering gene expression. In as much as blood levels are a strong reflection of dietary intake, it is proposed that an omega-3 FA biomarker, the omega-3 index (erythrocyte EPA+DHA) be considered at least a marker, if not a risk factor, for coronary heart disease, especially sudden cardiac death. The omega-3 index fulfils many of the requirements for a risk factor including consistent epidemiological evidence, a plausible mechanism of action, a reproducible assay, independence from classical risk factors, modifiability, and most importantly, the demonstration that raising tissue levels will reduce risk for cardiac events. For these and a number of other reasons, the omega-3 index compares very favourably with other risk factors for sudden cardiac death.
Keywords: Eicosapentaenoic acid, docosahexaenoic acid, biomarkers, risk factors, cardiovascular disease, coronary heart disease, sudden cardiac death, fish oils
Introduction
The American Heart Association [1], the European Society for Cardiology [2], the Scientific Advisory Committee on Nutrition (UK) [3], the Australian Health and Medical Research Council [4] and a host of other health agencies and professional organizations have issued recommendations for increased intakes of omega-3 fatty acids (FAs). These recommendations are based on strong evidence derived from a variety of scientific approaches linking dietary deficiency of long chain omega-3 FAs with risk for cardiovascular events, notably sudden death. These have been recounted in detail in several recent publications [1,5-11]. The primary purpose of this paper is to make a case for the use of a biomarker for omega-3 FA intake, “the omega-3 index,” in coronary heart disease (CHD) risk stratification. In so doing, the results of omega-3 epidemiological and interventional studies, and their apparent mechanisms of action will be briefly reviewed.
Omega-3 epidemiology
In order to summarize the fish/omega-3 ecological studies, He et al. [12] performed a meta-analysis of 13 cohorts including over 222,000 individuals followed for CHD death for an average of about 12 years. They found that the consumption of only one fish meal per week (vs. <1/mo) was associated with a statistically significant 15% reduction in risk. When subjects were classified into categories of increasing fish consumption (<1/mo, 1-3/mo, 1/wk, 2-4/wk, and ≥5/wk), those in the highest intake group enjoyed a 40% reduction in risk. Similar findings were reported for stroke [13].
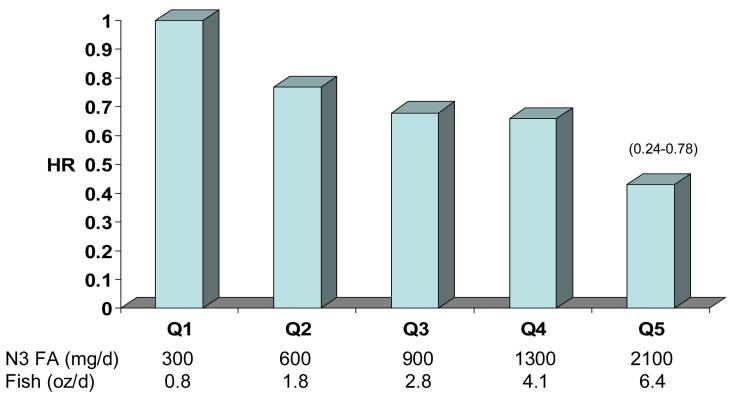
An inverse relation between fish intake and risk for CHD has also been recently reported in Greek [14] and in Japanese cohorts [15]. The latter study examined the association between fish (and omega-3 FA) intake and various CHD endpoints in 41, 578 Japanese men and women age 50-70 over a 10 year follow-up period [15]. The lowest quintile of intake was about 300 mg/d, which is about twice the median intake in the US [16]. At the high end, intakes averaged 2.4 g/d. The median intake in this cohort was about 900 mg/d, about 6-fold higher than US intakes. Across this intake gradient there was a significant reduction in risk for non-fatal coronary events and total myocardial infarctions (Figure 1). Hence, the intake of omega-3 FA at which benefits plateau is not yet defined.
Fig. 1.
Risk for MI by median omega-3 FA intake estimated from reported fish intake in the Japan Public Health Center-Based Study Cohort I. 41,578 subjects; 40-59 yrs; 10 yr f/u. Age, sex, smoking, alcohol, BMI, Hx HTN and diabetes, drug use for elevated cholesterol, educational level, sports in leisure time, and quintiles of dietary fruits, vegetables, sat fat, mono fat, n-6 fat, cholesterol and kcal. P for trend =0.02. Adapted from Iso et al. [15].
Omega-3 interventions
There have been several intervention studies of varying quality (see references [7,11]). The largest and most well-controlled was the GISSI Prevenzione study which tested the hypothesis that relatively small intakes of omega-3 FA (<1 g) could reduce risk for death from CHD in high risk patients. Over 11,000 post-myocardial infarction patients were randomized to either one capsule of omega-3 FA ethyl esters (Omacor, Pronova Biocare, Norway; 850 mg of EPA+DHA) or usual care and then followed them for 3.5 years. In this study, the risk for death from any cause was reduced by 20% and risk for sudden death by 45% in the supplement group [17].
To bring a perspective to the beneficial effects of omega-3 FA, Studer et al. [18] computed the relative reduction in risk for death from any cause in trials of anti-lipidemic drugs and lipid-lowering diets. These regimens are obviously prescribed not just to reduce serum lipid levels, but ultimately to reduce risk for death, typically from CHD. Over 137,000 patients receiving treatment for lipid disorders were compared to controls in a total of 97 studies. There were 35 trials with statins, 7 studies with fibrates, 8 with bile acid binding resins, 14 with omega-3 FA and 18 examining the effects of global dietary changes. Only two interventions were associated with significant reductions in total mortality: statins (risk ratio 0.87, 95% CI 0.81-0.94) and omega-3 FA (risk ratio 0.77, 95% CI 0.63-0.94). Although a fascinating analysis that underscores the fact that risk for serious adverse health outcomes can be reduced even without lowering cholesterol, one could argue that two studies included with the omega-3 group were not strictly omega-3 studies but overall dietary interventions. In these two studies [19,20], the active agent(s) cannot be identified with confidence because so many dietary variables differed between groups. Whether removing these studies from the omega-3 group would leave the latter still associated with reduced risk for death is not clear. Nevertheless, the preponderance of the data suggests that for most individuals, increasing the intake of long-chain omega-3 FA is a safe and inexpensive way to significantly reduce risk for CHD, especially sudden cardiac death.
Omega-3 mechanisms
The fundamental mechanism by which omega-3 FA appear to mitigate risk for CHD begins with the enrichment of membrane phospholipids with EPA and DHA. Once these long chain n-3 FA are resident in cell membranes, they may have at least four separate effects. The relative importance of each, their coordinated interaction and their sufficiency to explain the clinical observations have yet to be determined.
First, because of their highly unsaturated nature, they may alter membrane properties [21]. This can have the secondary effect of changing the microenvironment of transmembrane proteins (e.g., receptors) altering the manner in which they interact with their ligands [22]. Altering membrane FA composition can also affect the ability of membrane-associated proteins to actually associate with the membrane and consequently to interact with other multi-protein complexes involved with cell signaling systems [23]. In addition, a variety of cell stressors (e.g., inflammatory mediators) interact with transmembrane receptors and subsequently initiate intracellular G-protein linked responses, one of which is the activation of phospholipase A2 (PLA2). This enzyme hydrolyzes long-chain omega-6 and omega-3 FA esterified to inner leaflet phospholipids, liberating them and making them available for conversion to a wide variety of eicosanoids via cyclo-oxygenase, lipoxygenase, and cytochrome P-450 mono-oxygenases [24]. These molecules powerfully influence cellular metabolism. PLA2-liberated omega-3 FA may directly modify the activity of ion channel themselves, resulting in altered resting membrane potentials [25]. Finally, intracellular omega-3 FA are also able to serve as ligands for a variety of nuclear receptors [e.g., peroxisome proliferation activated receptors (PPARs), sterol receptor element binding protein (SREBP)-1c, retinoid X receptor, farnesol X receptor, and hepatocyte nuclear factor-α [26,27]] which impact inflammatory responses and lipid metabolism.
Presumably as a consequence of these membrane effects, omega-3 FA can diminish the activity of inflammatory cells and reduce levels of certain inflammatory mediators [24], which may ultimately result in reduced arterial plaque fragility [28]. Mild effects on blood pressure [29] are likely to be the result of an improvement in endothelial function [30]. The decrease in serum triglycerides that is produced by intakes of 3-4 g/d of EPA+DHA [31] appears to be secondary to increased hepatic beta-oxidation and decreased lipogenesis [32], which themselves are the result of modulation of the nuclear receptor actions noted above. Although the precise mechanisms by which omega-3 FA reduce risk for cardiac events are not known in detail, their presence in membranes and the coordinated downstream effects outlined here undoubtedly play a role.
Omega-3 biomarkers
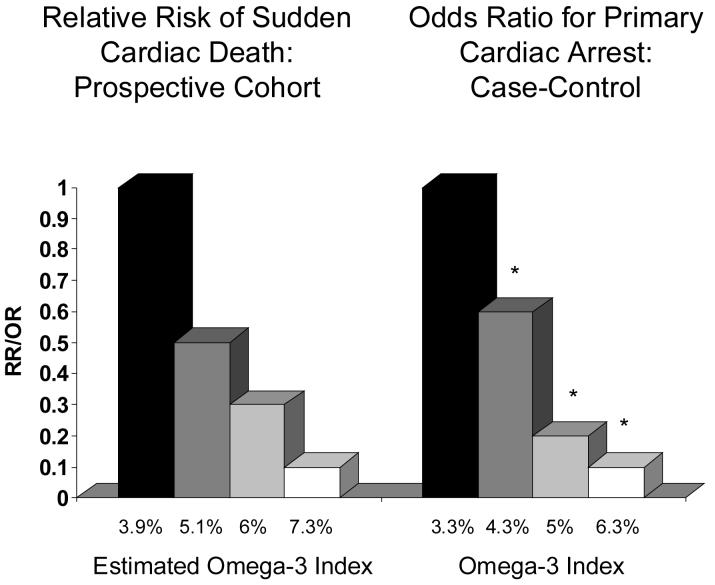
Another approach to linking omega-3 FA (and not just fish) with CHD risk has been to examine the relationships between omega-3 biomarkers and risk. Two studies are particularly relevant in this regard. Siscovick et al. [33] obtained blood samples from 80 adults experiencing primary cardiac arrest in the Seattle area and from 108 healthy matched controls. The cases did not have known CHD at the time of their events. The percent of total FA in red blood cell (RBC) membranes present as EPA+DHA was determined in these samples and related to the odds of being in the primary cardiac arrest group. Albert et al. [34] conducted a prospective case control study within the Physicians' Health Study. Healthy male physicians (n=14,916) were recruited and provided baseline blood samples between 1982 and 1984. Over the next 17 years, 94 men experienced sudden cardiac death. Whole blood long-chain omega-3 levels in the cases were compared to those of 184 matched controls. In both this and the Siscovick study, multivariable-adjusted odds for sudden cardiac death were reduced by 90% in those subjects with the highest blood omega-3 levels compared with those with the lowest levels (Figure 2). These studies both concluded that increased omega-3 FA intakes (as reflected by higher biomarker levels) were associated with a significant reduction in risk for sudden cardiac death. However, neither study directly recommended that blood omega-3 FA could be used as a risk factor.
Fig. 2.
The relationship between RBC EPA+DHA content and risk for primary cardiac arrest (right) or sudden cardiac death (left). The former data were derived from Siscovick et al. [33] from a population-based case control study, and the latter from Albert et al. [34] from a case-control study nested in the prospective Physicians' Health Study. The omega-3 index was estimated from the latter data set (which measured whole blood long-chain omega-3 FA content) using equations described by Harris and von Schacky [36].
Moving beyond sudden cardiac death, we recently tested the hypothesis that lower blood n-3 FA are associated with increased risk for acute coronary syndrome (ACSs). We analyzed the FA composition of whole blood from 94 ACS patients and 94 age- gender- and race-matched controls [35]. Omega-3 FA associations with ACS were assessed using multivariable models adjusting for smoking status, alcohol use, diabetes, body mass index, serum lipids, and history of myocardial infarction or revascularization. Whole EPA+DHA content was 29% lower in cases than controls (1.7±0.9 vs. 2.4±1.4%, p<0.001). Thus, low blood EPA+DHA content was an independent predictor of increased risk for ACS.
The omega-3 index
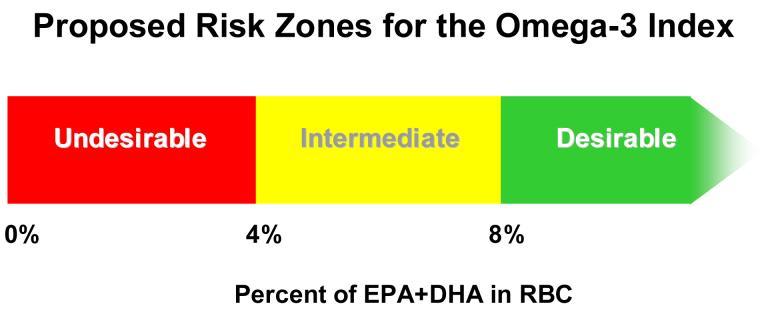
Clemens von Schacky and I recently proposed that the “omega-3 index” (EPA+DHA as a percent of total RBC FA) be considered a new risk factor for death from CHD [36]. We also suggested preliminary targets or cut-points to segregate those at low, intermediate and high risk based on a combination of a survey of the literature and correlations between intakes and omega-3 index levels determined experimentally. We estimated that a cardioprotective target level for the omega-3 index appeared to be about 8%, and the level associated with the increased risk for CHD death was <4% (Figure 3). These proposed cutpoints have yet to be prospectively tested, however. We recommended measuring the EPA+DHA content of RBC membranes (as opposed to whole plasma or plasma phospholipids) for the reasons outlined in Table 1.
Fig. 3.
Proposed risk zones for the omega-3 index (RBC EPA+DHA). Proposed cutpoints were estimated from a review of the literature [36].
Table 1.
Omega-3 index (and other omega-3 biomarkers) as potential risk markers
|
From biomarker to risk factor
There are several requirements that a putative risk factor/marker must meet in order to be clinically useful [37] (Table 1). The omega-3 index, even at this early stage of development, appears to fulfill many. The epidemiological data, both between and within populations, from prospective cohort and case-control studies are strong. A relationship between membrane EPA+DHA levels and risk for sudden cardiac death is biologically plausible. Currently, the most likely mechanism by which they appear to operate is via a reduction in myocardial susceptibility to lethal arrhythmias [25,38]. In addition, EPA+DHA may enhance plaque stability [28], and may be anti-atherosclerotic [39,40] via a variety of other mechanisms [1]. The omega-3 index is modifiable by increasing the dietary levels of omega-3 FA, and it has been shown to correlate with human myocardial omega-3 FA content [41]. The omega-3 index also appears to be independent of other known risk factors. In the ACS case-control study described above, the omega-3 index was associated with ACS case status even after taking serum lipids, age, sex, and a history of CHD into account. The same applies to the studies by Siscovick et al. [33] and Albert et al. [34]. Future studies are still needed to explore the question of independence more thoroughly. Finally, perhaps the most important question to be asked of a putative risk factor is whether changing it alters disease outcomes. The intervention trials described above indicate that EPA+DHA may fulfill this critical criterion as well.
Omega-3 index compared with other CHD risk factors
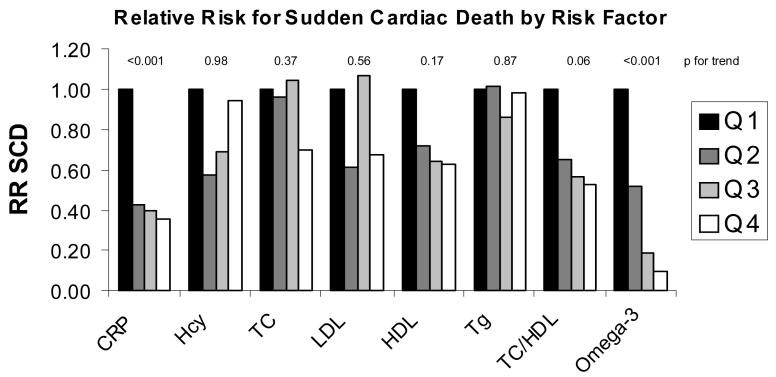
Using data from the Physicians' Health Study, Albert et al. [34,42] published the relative risk for sudden cardiac death across quartiles of several risk factors (Figure 4). Only two risk factors demonstrated statistically significant trends after controlling for age and smoking status: C-reactive protein and blood omega-3 FA content (which is highly correlated with the omega-3 index). Unfortunately, neither of these was adjusted for the other, nor was either adjusted for homocysteine or other lipid/lipoproteins classes. Thus, whether either of these adds predictive value beyond the other cannot be deduced from this analysis. Nevertheless, the omega-3 index was clearly related to risk in a dose dependent manner which was not the case for CRP. In addition, the risk reduction at the highest levels of the omega-3 index (90%) was greater than that associated with the lowest levels of CRP (65%). Thus, for the case of sudden cardiac death (which is responsible for about half of all CHD deaths [43] and is the first- and last- symptom of CHD in about 1/3rd of cases [44]), the omega-3 index may be more informative than any other known risk factor.
Fig. 4.
The multivariable-adjusted relative risk for sudden cardiac death (RR SCD) by quartile of blood omega-3 FA compared to other, more traditional blood-borne risk factors. The quartiles at highest risk (black bars) are set at a relative risk of 1.0 for the presumed highest-risk levels of each marker. Each subsequent lighter bar represents the risk at each decreasing (or, for HDL and omega-3 FA, increasing) quartile. Adapted from the Physicians' Health Study, Albert et al. [34,42]. CRP=C reactive protein; Hcy=homocysteine; TC=total cholesterol; LDL=low density lipoprotein cholesterol; HDL=high density lipoprotein cholesterol; Tg=triglycerides.
Conclusion
The potential for the omega-3 index (or any validated marker of tissue omega-3 FA status) to add clinically-important prognostic information regarding risk for death from CHD is significant. The omega-3 index has been validated as a surrogate for myocardial omega-3 FA composition in the human and as such reflects the omega-3 status of the most critical organ. It can be used to both assess baseline omega-3 status and to check for compliance with recommendations to increase omega-3 intake. Altering the omega-3 index is simple, safe and inexpensive and has been shown in randomized trials to reduce risk for CHD death. The widespread clinical implementation of the omega-3 index will allow clinicians to detect omega-3 “insufficiency”, to better stratify patients with respect to risk for SCD, and could ultimately contribute to a reduced burden of CHD.
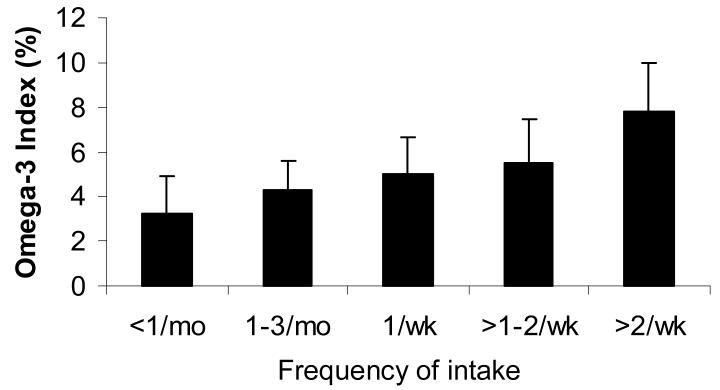
Fig. 5.
RBC EPA+DHA by category of reported consumption of tuna or non-fried fish per month. Groups differed by ANOVA (p<0.0001), with the highest RBC EPA+DHA group differing significantly from all others (p<0.05), and the second highest differing from the first (p<0.05) by Tukey's post-hoc test. Reprinted from Sands et al. [45] with permission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Manger C,V, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–10. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 3. http://www.food.gov.uk/news/newsarchive/2004/jun/fishreport2004.
- 4. http://www.nhmrc.gov.au/publications/synopses/n35syn.htm.
- 5.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Schacky CV, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.08.019. in press. [DOI] [PubMed] [Google Scholar]
- 7.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Harris WS, Assaad B, Poston WC. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary artery disease. Am J Cardiol. 2006;98:19i–26i. doi: 10.1016/j.amjcard.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JG, Stone NJ. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:39i–49i. doi: 10.1016/j.amjcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:50i–60i. doi: 10.1016/j.amjcard.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson TA. Secondary prevention of coronary artery disease with omega-3 fatty acids. Am J Cardiol. 2006;98:61i–70i. doi: 10.1016/j.amjcard.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 12.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–11. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Longstreth WT, Jr., Lemaitre RN, Manolio TA, Kuller LH, Burke GL, Siscovick DS. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–6. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagiotakos DB, Pitsavos C, Zampelas A, Chrysohoou C, Griffin BA, Stefanadis C, Toutouzas P. Fish consumption and the risk of developing acute coronary syndromes: the CARDIO2000 study. Int J Cardiol. 2005;102:403–9. doi: 10.1016/j.ijcard.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 16.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83:1526S–35S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 17.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E in 11,324 patients with myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 18.Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–30. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- 19.de Lorgeril M, Salen P, Martin JL, Renaud S, Monjaud I, Mamelle N, Delaye J. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 20.Singh RB, Dubnov G, Niaz MA, Ghosh S, Singh R, Rastogi SS, Manor O, Pella D, Berry EM. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–61. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 21.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 22.Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004;15:700–6. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–13. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 25.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2006;107:2646–52. doi: 10.1161/01.CIR.0000069566.78305.33. 2006. [DOI] [PubMed] [Google Scholar]
- 26.Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83:1520S–5S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- 28.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–85. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 29.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJH, Lewis MJ. Dietary Supplementation With Marine Omega-3 Fatty Acids Improve Systemic Large Artery Endothelial Function in Subjects With Hypercholesterolemia. J Am Coll Cardiol. 2000;35:265–70. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 31.Harris WS. n-3 Fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(suppl):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 32.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–93. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 33.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. J Am Med Assoc. 1995;274:1363–7. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 34.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 35.Harris WS, Reid KJ, Sands SA, Spertus JA. Blood omega-3 and trans fatty acids in middle-aged acute coronary syndrome patients. Am J Cardiol. 2007 doi: 10.1016/j.amjcard.2006.08.013. in press. [DOI] [PubMed] [Google Scholar]
- 36.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Manolio T. Novel risk markers and clinical practice. N Eng J Med. 49:1587–9. doi: 10.1056/NEJMp038136. 32003. [DOI] [PubMed] [Google Scholar]
- 38.Siscovick DS, Lemaitre RN, Mozaffarian D. The fish story: a diet-heart hypothesis with clinical implications: n-3 polyunsaturated fatty acids, myocardial vulnerability, and sudden death. Circulation. 2003;107:2632–4. doi: 10.1161/01.CIR.0000074779.11379.62. [DOI] [PubMed] [Google Scholar]
- 39.von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary w-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–62. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 40.Erkkila AT, Lichtenstein AH, Mozaffarian D, Herrington DM. Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr. 2004;80:626–32. doi: 10.1093/ajcn/80.3.626. [DOI] [PubMed] [Google Scholar]
- 41.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplant patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 42.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–9. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 43.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 44.Kannel WB, Wilson PW, D'Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–12. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 45.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 46.Dewailly E, Blanchet C, Lemieux S, Sauve L, Gingras S, Ayotte P, Holub BJ. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- 47.Davidson M, Bulkow LR, Gellin BG. Cardiac mortality in Alaska's indigenous and non-Native residents. Int J Epidemiol. 1993;22:62–71. doi: 10.1093/ije/22.1.62. [DOI] [PubMed] [Google Scholar]
- 48.Kromann N, Green A. Epidemiological studies in the Upernavik District, Greenland. Acta Med Scand. 1980;208:401–6. [PubMed] [Google Scholar]
- 49.Zhang J, Sasaki S, Amano K, Kesteloot H. Fish consumption and mortality from all causes, ischemic heart disease, and stroke: an ecological study. Prev Med. 1999;28:520–9. doi: 10.1006/pmed.1998.0472. [DOI] [PubMed] [Google Scholar]
- 50.Burchfiel CM, Reed DM, Strong JP, Sharp DS, Chyou P-H, Rodriguez BL. Predictors of myocardial lesions in men with minimal coronary atherosclerosis at autopsy. The Honolulu Heart Program. Ann Epidemiol. 1996;6:137–46. doi: 10.1016/1047-2797(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 51.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–9. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez BL, Sharp DS, Abbott RD, Burchfiel CM, Masaki K, Chyou PH, Huang B, Yano K, Curb JD. Fish intake may limit the increase in risk of coronary heart disease morbidity and mortality among heavy smokers. The Honolulu Heart Program. Circulation. 1996;94:952–6. doi: 10.1161/01.cir.94.5.952. [DOI] [PubMed] [Google Scholar]
- 53.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Eng J Med. 1997;336:1046–53. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 54.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–16. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 55.Oomen CM, Feskens EJ, Rasanen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151:999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- 56.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–82. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 57.Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish Consumption and Risk of Sudden Cardiac Death. JAMA. 1998;279:23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 58.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 59.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–7. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 60.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–7. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 61.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. N-3 polyunsaturated fatty acids, fatal ischemic heart disease and non-fatal myocardial infarction in older adults. The Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 62.Guallar E, Hennekens CH, Sacks FM, Willett WC, Stampfer MJ. A Prospective Study of Plasma Fish Oil Levels and Incidence of Myocardial Infarction in U.S. Male Physicians. J Am Coll Cardiol. 1995;25:387–94. doi: 10.1016/0735-1097(94)00370-6. [DOI] [PubMed] [Google Scholar]
- 63.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 64.Erkkila AT, Lehto S, Pyorala K, Uusitupa MI. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78:65–71. doi: 10.1093/ajcn/78.1.65. [DOI] [PubMed] [Google Scholar]
- 65.Simon JA, Hodgkins ML, Browner WS, Neuhaus JM, Bernert JT, Jr., Hulley SB. Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol. 1995;142:469–76. doi: 10.1093/oxfordjournals.aje.a117662. [DOI] [PubMed] [Google Scholar]
- 66.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–61. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 67.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 68.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 69.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med. 1990;323:439–45. doi: 10.1056/NEJM199008163230703. [DOI] [PubMed] [Google Scholar]
- 70.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]
- 71.Giltay EJ, Geleijnse JM, Schouten EG, Katan MB, Kromhout D. High Stability of Markers of Cardiovascular Risk in Blood Samples. Clin Chem. 2003;49:652–5. doi: 10.1373/49.4.652. [DOI] [PubMed] [Google Scholar]
- 72.Hodson L, Skeaff CM, Wallace AJ, Arribas GL. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clin Chim Acta. 2002;321:63–7. doi: 10.1016/s0009-8981(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 73.Tynan MB, Nicholls DP, Maguire SM, Steele IC, McMaster C, Moore R, Trimble ER, Pearce J. Erythrocyte membrane fatty acid composition as a marker of dietary compliance in hyperlipidaemic subjects. Atherosclerosis. 1995;117:245–52. doi: 10.1016/0021-9150(95)05578-k. [DOI] [PubMed] [Google Scholar]
- 74.Marangoni F, Colombo C, Galli C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: applicability to nutritional and epidemiological studies. Anal Biochem. 2004;326:267–72. doi: 10.1016/j.ab.2003.12.016. [DOI] [PubMed] [Google Scholar]