Summary
Esterification of cidofovir (CDV), an antiviral nucleoside phosphonate, with alkyl or alkoxyalkyl groups increases antiviral activity by enhancing cell uptake and conversion to CDV diphosphate. Hexadecyloxypropyl-CDV (HDP-CDV) has been shown to be 40 to 100 times more active than CDV in vitro in cells infected with herpes group viruses, variola, cowpox, vaccinia or ectromelia viruses. Since the first phosphorylation of CDV may be rate limiting, we synthesized the hexadecyloxypropyl-phosphate (HDP-P-) and octadecyloxyethyl-phosphate (ODE-P-) conjugates of CDV and phosphonomethoxy-ethyl-adenine (PMEA, adefovir). We tested the CDV analogs in cells infected with human cytomegalovirus, herpes simplex virus, cowpox virus and vaccinia virus; the analogs of PMEA were tested in cells infected with the human immunodeficiency virus, type 1. In general, the alkoxyalkyl-phosphate conjugates of CDV were substantially more active than CDV. HDP-P-CDV and ODE-P-CDV were 4.6 to 40 times more active against HCMV and 7 to 30 times more active against cowpox and vaccinia in vitro. Although the compounds of this type were more cytotoxic than the unmodified bases, their selectivity for virally infected cells was generally greater than the parent nucleotides except that HDP-P-PMEA showed little or no selectivity in HIV-1 infected MT-2 cells. Although the new compounds with an interposed phosphate were generally less active that the corresponding alkoxyalkyl esters of CDV and PMEA, the present approach provides a possible alternative method for enhancing the antiviral activity of drugs of this class.
Acyclic nucleoside phosphonates are well known and are in clinical use as antiviral agents for cytomegalovirus (cidofovir, CDV), HIV (tenofovir) and hepatitis B (adefovir, PMEA) (Holy, 2003). The limitations of acyclic nucleoside phosphonates relate to their poor oral bioavailability and nephrotoxicity (Cundy, 1999) In general, the uptake of nucleoside phosphonates such as CDV into target cells is poor because of the dual negative charges on the phosphonate moiety and the slow uptake by fluid phase endocytosis (Conelley et al, 1993; Aldern et al, 2003). Once in the cell, they require two subsequent anabolic phosphorylations to achieve activity as their diphosphates (Ho et al, 1992).
We showed previously that the antiviral activity of CDV could be markedly increased by conversion to HDP-CDV (Beadle et al, 2002; Kern et al, 2002). Furthermore, HDP-CDV is orally active in mice against lethal infections with ectromelia virus (Buller et al, 2004), vaccinia and cowpox virus infections (Quenelle et al, 2004), lethal murine cytomegalovirus (CMV) infection (Kern et al, 2004) as well as in human CMV (HCMV) infection in SCID-hu mice (Bidanset et al, 2004). Cellular uptake studies of [2-14C]CDV and HDP-[2-14C]CDV in MRC-5 cells indicated that cellular drug and metabolite levels were many fold higher with HDP-CDV. In cells exposed to HDP-CDV for 48 hr, cellular levels of the metabolites for CDV, CDVp and CDVpp were 702, 71 and 184 picomoles/flask, respectively (Aldern et al, 2003). Similar results were reported in cellular metabolism studies of CDV in MRC-5 cells (Ho et al, 1992). Thus, it appears that the first phosphorylation may be the rate-limiting step in activation of CDV to CDVpp. Bypassing the first phosphorylation step from CDV to CDVp could increase the activity of antiviral nucleoside phosphonate drugs. Therefore, it would be of interest to see if alkoxyalkyl phosphosphate conjugates of CDV also exhibit greater antiviral activity due to enhanced cell uptake and favorable cellular metabolism.
To address this question, we synthesized alkoxyalkyl phosphate adducts of (S)-9-[3-hydroxy-2-(phosphonomethoxy)-propyl]cytosine (cidofovir, CDV) and phosphonomethoxyethyladenine (PMEA) and tested the former for antiviral activity in cells infected with HCMV, HSV-1, vaccinia and cowpox and the latter in cells infected with HIV-1.
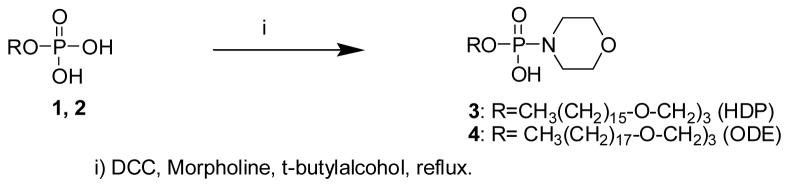
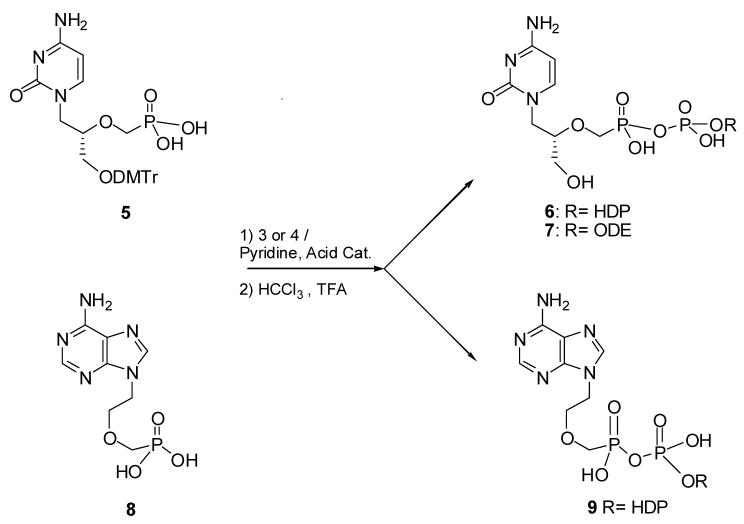
The nucleoside phosphonate-phosphate conjugates were prepared as depicted in Schemes 1 and 2. Scheme 1 outlines the synthesis of alkoxyalkyl phosphomorpholidates 3 and 4. The alkoxyalkyl phosphomorpholidates were coupled to the nucleoside phosphonates as shown in Scheme 2. (S)-1-(3-hydroxy-2-phosphono-methoxypropyl)cytosine (HPMPC) was treated with dimethoxytritylchloride in DMSO by the method of Otmar et al (1999) to give the intermediate 5 that was condensed with HDP-phosphate morpholidate 3 and 4 in pyridine, tributylamine and catalytic acetic acid at room temperature. Finally, hydrolysis with TFA in CHCl3 gave compounds 6, hexadecyloxypropyl-P-cidofovir (HDP-P-CDV), and 7, octadecyloxyethyl-phospho-cidofovir (ODE-P-CDV). Compound 9 was prepared from the condensation of compound 8, 9-(2-phosphonylmethoxyethyl)adenine (PMEA), and compound 3 in pyridine and acetic acid as catalyst. Detailed synthetic methods are as follows.
Scheme 1.
Synthesis Of Alkoxyalkyl-Phosphomorpholidates
Scheme 2.
Synthesis Of Alkyoxyalkyl Phosphate Esters Of Acyclic Nucleoside Phosphonates
2-(octadecyloxy)ethyl dihydrogen phosphate (2): To a cold solution of phosphorus oxychloride, 3 ml (32 mmol) in THF was added dropwise a solution of 2-octadecyloxy-1-ethanol (5 g,16 mmol) and triethylamine (4.4 ml, 32 mmol) in THF, while the temperature was maintained bellow 20 °C. The mixture was kept an additional hour, water was added and the stirring continued overnight followed by extraction with ethyl ether. The crude solid from the ether layer was recrystallized from hexane to give 2 as a white solid in 72% yield.
2-(octadecyloxy)ethyl hydrogen morpholinophosphonate (4): To a solution of 3 g (7.6 mmol) of 2-(octadecyloxy)ethyl phosphate in tert-butyl alcohol was added 2 g (24 mmol) of morpholine and 5.8 g (30 mmol) of DCC, added in four portions and refluxed over 48 h. Ethyl ether was added to the mixture, then it was filtered, and the filtrate concentrated to give 4 as an oil. Mass spectrum: (ESI) m/z 462 (M-H)-
3-(hexadecyloxy)propyl dihydrogen phosphate (1): Compound 1 was prepared following the same procedure described above except that 3-(hexadecyloxy)-1-propanol was substituted for 2-octadecyloxy-1-ethanol. The yield was 71%.
3-(hexadecyloxy)propylhydrogenmopholinophosphonate (3): HDP-phosphonomorpholidate was prepared using the procedure described in example 1 using 1.92g (15 mmol) of 3-(hexadecyloxypropyl) dihydrogen phosphate, 1.3g (15 mmol) of morpholine and 35 mmol of DCC. 3 was obtained as an oil. Mass spectrum: (ESI) m/z 449 (M-H)−.
(S)-1-(3-(4,4′-dimethoxytrityloxy)-2-phosphonomethoxypropyl)cytosine (5): (S)-1-(3-hydroxy-2-phosphonomethoxypropyl)cytosine dihydrate (free acid, 0.25g, 0.75 mmol) was mixed with methanol and 3 ml of tributylamine. After solution was achieved, solvents were removed and the solid residue dissolved in DMSO; 0.9 g of tributylamine and 0.7 g of dimethoxytritylchloride was added and the mixture was stirred overnight at room temperature. Solvents were removed and the solid residue was recrystallized from ethyl acetate and dried to obtain 0.35g (91%) of the product 5 as a white solid. MS (ESI) m/z 943 (M-H)−.
(1-(4-aminooxopyrimidin-1(2H)-yl)-3-hydroxypropan-2-yloxy)methyl-phosphonic(3-hexadecyloxy)propylphosphoric) anhydride (HDP-P-CDV, 6): To a suspension of 5, 0.1 g (0.17 mmol ) in pyridine was added 0.1 ml of tributylamine. After solution was achieved, 1g of 3 in pyridine and 0.1 ml of acetic acid were added and the solution was stirred for 48h. Solvents were removed and the residue was mixed with a solution of CHCl3 and TFA (5:0.5) and stirred at room temperature. Solvents were removed and the crude mixture was purified by flash chromatography using silica gel and eluting with 70:30:3:3 (CHCl3:methanol:ammonium hydroxide:water) to obtain 0.04g (40 %) of 6 as a white solid. MS (ESI) m/z 642 (M-H)−. 1H NMR 300MHz (DMSO) δ ppm: 7.47 (d,1H, 6.9Hz), 5.66 (d, 1H,7.2Hz), 3.8-3.1 (mm, 16H), 1.7 (m, 2H), 1.4 (m,2H), 1.2 (s, 26H), 0.8 (t,3H). 31P NMR (DMSO-d6) 12.5 (s, P1), 0.4(s, P2).
(1-(4-aminooxopyrimidin-1(2H)-yl)-3-hydroxypropan-2-yloxy)methyl-phosphonic-(3-octadecyloxy)ethyl phosphoric) anhydride (ODE-P-CDV, 7): To a suspension of 5 (0.14 g, 0.17 mmol ) in pyridine was added 0.1 ml of tributylamine followed by a solution of 1g of 4 in pyridine and 0.1 ml of acetic acid were added and stirred for 48h. Solvents were removed and the residue was mixed with a solution of CHCl3:TFA in (5:0.5) and stirred at room temperature. After complete deprotection, the mixture was purified by column chromatography using silica gel and eluting with 70:30:3:3 (CHCl3:methanol:amonia:water) to obtain 0.03g (20 %) of 7 as a white solid. MS (ESI) m/z 654 (M-H)−. 1H NMR 300MHz (CDCl3/CD3OD) δ ppm: 6 (d,1H, J=8Hz), 5.8 (d, 1H,J=7.2Hz), 4.2-3.4 (mm, 16H), 1.7 (m, 2H), 1.58 (m,2H), 1.27 (s, 28H), 0.89 (t,3H). 31P NMR (CDCl3/CD3OD), 12.38 (m.P1), 5.6 (d.P2,J=25.5Hz).
(2-(6amino-9H-purin-9-yl)ethoxy)methylphosphonic (3-hexadecyloxy)-propylphosphonic)anhydride (HDP-P-PMEA, 9): 9-(2-phosphonylmethoxyethyl)-adenine (PMEA) 0.5 g was dissolved in pyridine and a solution of 1 g of 3 in pyridine was added along with 1 ml of acetic acid. The reaction mixture was heated at 40°C overnight. Solvents were removed and the oily residue was washed with 10% methanol in ethyl ether and filtered. The filtrate was concentrated and purified by column chromatography using silica gel and eluting with 25% methanol in dichloromethane to obtain 0.19g of 9 (17 %) as a white solid. Mass spectrum: (ESI) m/z 635(MH)−, 636(M+H)+. 1H NMR 300MHz (DMSO) δ 8.35 (s,1H), 8.09 (s,1H), 7.86 (bs,2H), 7.12 (bs,2h), 0.83 (s,3H). 31P NMR (DMSO) δ1.4 (d,P1,JPP= 24.32Hz), -12 (d,P2,JPP= 24.32Hz).
The effect of the various compounds on HIV replication was measured by p24 reduction as previously described (Hammond et al 2001). Drug effects on HCMV replication were assessed by plaque reduction assay in HFF cells as described previously (Beadle et al 2002; Wan et al 2005). Antiviral activity against HSV-1 was assessed in MRC-5 cells as previously reported using a DNA reduction assay (Beadle et al 2002). Drug activity against cowpox and vaccinia virus infected human foreskin fibroblast cells (HFF) was determined by plaque reduction assay (Keith et al, 2004) Cytotoxicity was assessed in HFF or MRC-5 cells by neutral red uptake and the CC50s determined as reported previously (Beadle et al 2002; Keith et al, 2004; Wan et al, 2005). The antiviral activity of the various compounds is expressed as the EC50, the concentration required to inhibit p24, plaques or viral DNA production by 50%.
Both HDP-P-CDV (6) and ODE-P-CDV (7) were more active than CDV with EC50 values of 0.26 and 0. 0.03 μM versus 1.2 μM for CDV in vitro against HCMV; this represents an increase of 5 to 40 fold (Table 1). Similar results were noted against HSV-1 with EC50 values for HDP-PCDV and ODE-P-CDV of 0.06 and 0.00002 μM versus 3.3 μM for CDV. Although the cytotoxicity of HDP-P-CDV and ODE-P-CDV was higher than that of CDV, the selectivity index for the former two compounds against HCMV and HSV-1 ranged from 770 to >400,000. However, when compared against the antiviral activity of the HDP- and ODE- esters of CDV (Beadle et al, 2002), HDP-P-CDV and ODE-P-CDV are less active with the exception of ODE-PCDV versus HSV-1 (Table 1). In cells infected with cowpox and vaccinia generally similar results were noted. Again, the antiviral activity of HDP-P-CDV and ODE-P-CDV was substantially greater than CDV against cowpox and vaccinia, but less than noted previously with HDP-CDV and ODE-CDV versus CDV (Kern et al, 2002; Keith et al, 2005).
TABLE 1.
ANTIVIRAL ACTIVITY OF CIDOFOVIR, ALKOXYALKYL AND ALKOXYALKYL PHOSPHATE CONJUGATES OF CIDOFOVIR AGAINST HERPESVIRUSES AND ORTHOPOXVIRUSES, IN VITRO
| EC50, μM | CC50, μM | ||||
|---|---|---|---|---|---|
| Compound | HCMV1 | HSV-12 | Cowpox3 | Vaccinia4 | |
| CDV | 1.2±0.43a,c | 3.3±3.7a | 42±5.4 b | 31±5.4 b | >317 |
| HDP-CDV | 0.0009±0.0001a | 0.0001±0.0001a | 0.5±0.3b | 0.6±0.4b | 31±4 |
| ODE-CDV | 0.0009±0.0001a | 0.001±0.002a | 0.2±0.2b | 0.2±0.1b | 18±3 |
| HDP-P-CDV | 0.26±0.20d | 0.06±0.02 | 6.2±3.3 | 3.2+0.5 | 77±4.4 |
| ODE-P-CDV | 0.03±0.04d | 0.00002±0.00002 | 0.9±0.5 | 1.1±1.1 | 9.5±4.3 |
Results expressed as EC50 and CC50 in μM and are the mean ± S.D. of three or more determinations unless otherwise indicated. Abbreviations: EC50, 50% effective concentration; CC50, 50% cytotoxic concentration; HDP-CDV, hexadecyloxypropyl-cidofovir; ODE-CDV, octadecyloxyethyl-cidofovir, HDP-P-CDV: 3-(hexadecyloxy)propyl-phospho-cidofovir (6), ODE-P-CDV: 2-(octadecyloxy)ethyl-phospho-cidofovir. Antiviral assays:
HCMV (AD-169) plaque reduction assay in HFF cells;
HSV-1 DNA reduction assay in MRC-5 Cells,
Cowpox Brighton, plaque reduction assay in HFF cells;
Vaccinia Copenhagen plaque reduction assay in HFF cells.
- data from Beadle et al, 2002;
- data from Kern et al, 2002;
– data from Wan et al 2005;
- mean ± S.D. of two determinations.
We prepared HDP-P-PMEA (HDP-P-adefovir) and assessed its antiviral activity in MT-2 cells infected with HIV-1lai by p24 reduction (Table 2). HDP-P-PMEA had an EC50 of 3 nanomolar against HIV-1 but its cytotoxicity in MT-2 cells was much greater than that of PMEA itself, 0.018 μM versus 157 μM. The selectivity index of HDP-P-PMEA was only 6 versus 121 for PMEA. However, HDP-PMEA, the hexadecyloxy-propyl ester of PMEA, had an EC50 of 0.2 nanomolar and a CC50 of 60 nanomolar. The selectivity index for HDP-PMEA was 3,000, twenty five times greater than that of PMEA (Table 2).
TABLE 2.
ANTIVIRAL ACTIVITY OF ALKOXYALKYL AND ALKOXYALKYL PHOSPHATE CONJUGATES OF PHOSPHONOMETHOXYETHYLADENINE AGAINST HIV-1, IN VITRO
| COMPOUND | HIV-1 EC50 | MT-2 CC50 | S.I. |
|---|---|---|---|
| PMEA | 1.30±0.70 (7) | 157±54(3) | 121 |
| HDP-PMEA | 0.00002±0.00003 (4) | 0.06±0.09(3) | 3,000 |
| HDP-P-PMEA | 0.003±0.005 (3) | 0.018±0.013(3) | 6 |
Results expressed as EC50 and CC50 in μM and are the mean ± S.D. of three or more determinations. Abbreviations: PMEA, Phosphonomethoxyethyadenine; HDP-PMEA, hexadecyloxypropyl- phosphonomethoxyethyadenine; HDP-P-PMEA, 3-(hexadecyloxy)propyl-phospho-phosphonomethoxy-ethyadenine; Assays: HIV-1Lai p24 reduction assay in MT-2 cells. CC50 is the micromolar concentration which reduces viable cell number by 50% in a propidium iodide staining assay.
In summary, our data suggest HDP-P-CDV and ODE-P-CDV are able to enter the cell more rapidly than CDV and are able to be metabolized to CDVpp. A phospholipase C type of enzyme might generate CDVp directly. However, HDP-P-CDV and ODE-P-CDV are less active than HDP-CDV and ODE-CDV indicating that their uptake and conversion to CDVpp is less extensive. Similar conclusions can be drawn with respect to PMEA, HDP-PMEA and HDP-PPMEA against HIV-1 (Table 2). Thus, interposition of an additional phosphate residue between the alkoxyalkyl and CDV or PMEA leads to greater in vitro activity against herpes viruses and poxviruses than the activity observed with the unmodified nucleotide. However, the alkoxyalkyl-phosphate esters of CDV are generally less active than the corresponding alkoxyalkyl esters.
Footnotes
Portions of this paper were presented in abstract form at the International Conference on Antiviral Research, April 11-14, 2005, Barcelona, Spain
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aldern KA, Ciesla S, Winegarden K, Hostetler KY. The increased antiviral activity of 1-O-hexadecyloxypropyl-cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 2003;63:678–681. doi: 10.1124/mol.63.3.678. [DOI] [PubMed] [Google Scholar]
- 2.Beadle JR, Rodriquez N, Aldern KA, Hartline C, Harden E, Kern ER, Hostetler KY. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication, in vitro. Antimicrob. Agents Chemother. 2002;46:2381–2386. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid prodrugs of cidofovir against experimental human cytomegalovirus infections. J. Virol. 2004;190(3):499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- 4.Buller RM, Owens G, Schriewer J, Melman L, Beadle JR, Hostetler KY. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318(2):474–81. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Connelly MC, Robbins BL, Fridland A. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in Vero cells. Biochem. Pharmacol. 1993;46:1053–1057. doi: 10.1016/0006-2952(93)90670-r. [DOI] [PubMed] [Google Scholar]
- 6.Cundy KC. Clinical pharmacokinetics of the antiviral nucleotide analogs cidofovir and adefovir. Clin. Pharmacokinet. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hammond JL, Koontz D, Bazmi HZ, Beadle JR, Hostetler SE, Kini GD, Aldern KA, Richman DD, Hostetler KY. Alkylglycerol prodrugs of phosphonoformate are potent in vitro inhibitors of nucleoside resistant HIV type 1 and select for resistance mutations that reverse AZT resistance. Antimicrob. Agents Chemother. 2001;45:1621–1628. doi: 10.1128/AAC.45.6.1621-1628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho HT, Woods KL, Bronson JJ, De Boeck H, Martin JC, Hitchcock MJM. Intracellular metabolism of the antiherpesvirus agent (S)-1-[3-hydroxy-2-(phosphonylmethoxyl) propyl]cytosine. Mol. Pharmacol. 1992;41:197–202. [PubMed] [Google Scholar]
- 9.Holy A. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharmaceutical Des. 2003;9(31):2567–2592. doi: 10.2174/1381612033453668. [DOI] [PubMed] [Google Scholar]
- 10.Hostetler KY, Kini GD, Beadle JR, Aldern KA, Gardner MF, Border R, Barshak L, Kumar R, Sridhar CN, Wheeler CJ, Richman DD. Lipid prodrugs of phosphonoacids: greatly enhanced antiviral activity of 1-O-octadecyl-sn-glycero-3- phosphonoformate in HIV-1, HSV-1 and HCMV-infected cells, in vitro. Antiviral Res. 1996;31:59–67. doi: 10.1016/0166-3542(96)00947-3. [DOI] [PubMed] [Google Scholar]
- 11.Keith KA, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication, in vitro. Antimicrob. Agents Chemother. 2004;48(5):1869–1871. doi: 10.1128/AAC.48.5.1869-1871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kern ER, C. Hartline C, Harden E, Keith K, Rodriguez N, Beadle JR, Hostetler KY. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern ER, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Quenelle DC. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48(9):3516–3522. doi: 10.1128/AAC.48.9.3516-3522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otmar M, Votruba I, Holy A. An alternative synthesis of HPMPC and HPMPA diphosphoryl derivatives. Collection Symposium Series 2 [Chemistry of Nucleic Acid Components] 1999:252–54. [Google Scholar]
- 15.Quenelle DC, Collins DJ, Hostetler KY, Beadle JR, Wan WB, Hostetler JR, Beadle WB, Wan, Kern ER. Oral treatment of cowpox and vaccinia infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan WB, Beadle JR, Hartline CB, Kern ER, Ciesla SL, Valiaeva N, Hostetler KY. Comparison of the antiviral activity of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 2005;49:656–662. doi: 10.1128/AAC.49.2.656-662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]