Abstract
Substantive evidence indicates that there are sex differences in the reinforcing effects of drugs and gonadal steroid hormones, such as estrogen and progesterone, likely contribute to these differences. Among females, subjective effects of drugs differ as a function of menstrual cycle phase. The purpose of the present study was to compare oral self-administration of phencyclidine (PCP) in female rhesus monkeys (Macaca mulatta) across different phases of the menstrual cycle. Since the 28-day menstrual cycle of nonhuman primates is similar to that of humans, this model could provide important evidence supporting the implication that changes in the levels of gonadal hormones across menstrual phases can alter a drug’s reinforcing effects. Oral self-administration of several concentrations of PCP (0.125, 0.25, and 0.5 mg/ml) was examined in three sexually mature female monkeys during 3-h experimental sessions. Menstrual cycle phase was determined by onset of menses, and verified by examining vaginal cytology. Overall, PCP self-administration was greater during the luteal phase, which is normally characterized by high levels of progesterone and moderate levels of estrogen, than the follicular phase, when levels of estrogen are increasing and progesterone levels are low. When examined within each phase, numbers of PCP deliveries were highest during the mid-luteal phase, compared to the early and mid-follicular phases. No differences in self-administration were observed between early and mid-follicular phases, but a significant difference in PCP deliveries was found between mid- and late luteal phases at the lowest concentration of PCP tested. The results from this study suggest that PCP’s reinforcing effects in female monkeys differ as a function of menstrual cycle phase.
Keywords: Menstrual cycle, Oral drug self-administration, Phencyclidine, Female, Rhesus Monkey
1. Introduction
A growing body of clinical and preclinical research has revealed a sexually dimorphic pattern in the effects of abused substances including their reinforcing effects (Lynch et al., 2002; Roth et al., 2004; Sofuoglu et al., 1999; White et al., 2002). Females appear to be more sensitive to the positive effects of drugs than males, and the basis of the heightened susceptibility of females to abuse drugs may be attributable to the circulating ovarian hormones estrogen and progesterone (Carroll et al., 2004; Terner and de Wit, 2006). The female menstrual cycle is characterized by fluctuations of steroid hormones across a 28-day cycle, beginning with menses on day 1 of the cycle. During the follicular phase, which is typically defined as the post-menses period lasting until ovulation (days 3–14 following menses onset), serum levels of estrogen increase and levels of progesterone remain low. The period following ovulation and lasting until the onset of menses (days 17–28 following menses onset) is referred to as the luteal phase, during which there is a sustained elevation of progesterone in addition to a moderate increase in estrogen.
In humans, subject-rated effects produced in response to drug administration have been shown to differ between the luteal and follicular phases. During the luteal phase of the menstrual cycle, women report reduced positive effects of cocaine compared to the follicular phase (Evans et al., 2002; Sofuoglu et al., 1999). Similarly, Justice and de Wit (1999) found that during luteal phase, women report significantly lower levels of “euphoria”, “high”, “liking” and “wanting” for amphetamine compared to the follicular phase, a finding they attributed to the effects of progesterone. In a subsequent study White et al. (2002) found that positive subject-rated effects of amphetamine were positively correlated with levels of salivary estrogen and negatively correlated with salivary progesterone levels during the follicular and luteal phases, respectively.
The role of ovarian hormones in mediating subjective and reinforcing effects of drugs is supported by studies examining effects of exogenously administered estrogen and progesterone. Justice and de Wit (2000a) found that estradiol given to women during the early follicular phase increases some, but not all, positive subject-rated effects of amphetamine. Others have administered progesterone during the follicular phase, when endogenous levels are negligible, and found that it decreases physiological and positive subject-rated effects of cocaine in women (Evans and Foltin, 2006a; Sofuoglu et al., 2004). Menstrual cycle phase also affects the severity of withdrawal from drug use. Nicotine withdrawal symptomology is more pronounced in women abstaining from smoking during the luteal phase (Perkins et al., 2000) or the late luteal phase (Allen et al., 2000; Allen et al., 1996) than those initiating abstinence during the follicular phase. Presentation of nicotine-associated cues induces greater levels of self-reported craving during the luteal phase than when presented during the follicular phase (Franklin et al., 2004).
Most of the preclinical evidence regarding the role of ovarian hormones in drug self-administration comes from studies that have examined responding across the estrous cycle in rats and those that have manipulated gonadal hormones surgically or pharmacologically. During the rat estrus phase, when estrogen and progesterone levels begin to decline, rats respond more for infusions of cocaine under a progressive ratio schedule of reinforcement (Hecht et al., 1999; Roberts et al., 1989), while responding maintained by a non-drug reinforcer remains unchanged (Hecht et al., 1999). Ongoing cocaine self-administration becomes highly dysregulated during estrus compared to other phases of the estrous cycle (Lynch et al., 2000). In rats, removal of endogenous ovarian hormones by ovariectomy or blocking estrogen’s actions with the antagonist, tamoxifen, reduces the acquisition of cocaine self-administration (Hu et al., 2004; Jackson et al., 2006; Lynch et al., 2001). Ovariectomy also reduces, while estrogen treatment enhances, cocaine-induced reinstatement of drug seeking (Larson et al., 2005). Progesterone administered to ovariectomized rats that are treated with estrogen replacement reduces acquisition of cocaine self-administration (Jackson et al., 2006), and in intact rats, progesterone decreases the magnitude of cocaine-induced reinstatement (Anker et al., 2006). The effects of exogenously administered progesterone on cocaine self-administration have also been examined in female rhesus monkeys. Mello et al (2005) demonstrated that progesterone dose-dependently decreases the reinforcing effects of cocaine, a finding consistent with those reported in the human literature.
Although there is much evidence implicating the role of ovarian steroid hormones in psychostimulant-mediated responses, relatively few studies have examined the influence of ovarian hormones on the reinforcing effects of other drugs of abuse. For example, in contrast to what has been found for cocaine and amphetamine, most clinical studies investigating menstrual cycle phase and the subject-rated effects of alcohol (Holdstock and de Wit, 2000; Nyberg et al., 2004), nicotine (Marks et al., 1999; Pomerleau et al., 1994), and marijuana (Griffin et al., 1986; Lex et al., 1984) have failed to show consistent differences, if any, across menstrual cycle. Thus, evidence within the existing literature suggests that whether or not ovarian hormones impact the reinforcing and subject-rated effects of drugs could depend on the drug under investigation.
Sex differences in the reinforcing effects of PCP have been shown in recent studies. For example, female rhesus monkeys exceed males in the rate of acquisition of oral PCP self-administration as demonstrated by an earlier preference for PCP over water (Carroll et al., 2000). Additionally, females respond differently to pharmacological (Cosgrove and Carroll, 2004) and behavioral (Cosgrove and Carroll, 2003) treatments of PCP self-administration compared to males. Females also escalate their intake of PCP more rapidly than males under extended access conditions (Carroll et al., 2005). Sex differences have been demonstrated in behavioral dependence upon on orally self-administered PCP. Perry et al (2006) found that males exhibit a stronger and more prolonged disruption in food-maintained behavior after withdrawal from PCP than females.
The purpose of the present study was to examine the reinforcing effects of several concentrations (0.125, 0.25, 0.5 mg/ml) of PCP across the menstrual cycle in rhesus monkeys. The menstrual cycle of the nonhuman primate is 28-days in duration, similar to humans, and the duration and patterns of hormone fluctuation are comparable to those found in humans (Goodman and Hodgen, 1983; Pohl and Knobil, 1982). In the present study, menstrual cycle was monitored by observing onset of menses and menstrual phases were verified according to vaginal cytology (Mauro et al., 1970; Stute et al., 2004). PCP self-administration was examined during the follicular phase (days 3–10 following menses onset) and the luteal phase (the final 8 days of a cycle prior to the observation of menses). To further examine whether self-administration varied within each phase, follicular and luteal phases were subdivided into early follicular (days 3–6) and mid-follicular (days 7–10) phases, and mid-luteal (the first 4 of the last eight days of the cycle) and late luteal (last 4 days of the cycle) phases. Mid-follicular and mid-luteal phases have previously been shown to correspond with increases in serum levels of estrogen and progesterone, respectively, in monkeys (Evans and Foltin, 2006b; Roth et al., 2005).
2. Methods
2.1 Subjects
Three adult female rhesus monkeys (Macaca mulatta; 6.3 – 7.8 kg) with extensive histories of PCP self-administration served as subjects. Monkeys were maintained at 85% of their free-feeding weights, and they were fed measured allotments of monkey chow (Harland Teklad Monkey Chow; Bartonville, IL) and fresh fruit and/or trail mix following their daily experimental sessions. Monkeys were weighed monthly and food amounts were adjusted if needed to maintain them at 85% of their free-feeding weights. They were individually housed in temperature- and humidity-controlled colony rooms on a 12-h light/dark schedule with lights on at 0600 h. All procedures and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Laboratory practices were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2003). The health of the monkeys was monitored daily by research staff and examined several times each week by veterinarians.
2.2 Apparatus
Monkeys were housed in individual, custom-made stainless steel cages (86 cm in width × 84 cm in height × 78 cm in depth; Suburban Surgical, Chicago, IL) consisting of solid back and side walls, a barred front door, grid floors and a primate perch. One of the side walls was modified to accommodate an intelligence panel with two brass spouts (1.2 cm in diameter) that extended 2.7 cm into the cage through circular cutouts in the wall about 45 cm above the cage floor. The two spouts were spaced equidistantly from the center and sides of the cage. Two green-colored stimulus lamps were located above the spouts. The stimulus lights flashed (10 Hz) to indicate drug availability or were continuously lit to indicate water availability. Each spout was mounted on clear Plexiglas surrounded by two green and two white stimulus lamps that were illuminated upon lip contact when PCP or water was being delivered, respectively. When the appropriate number of lip contact responses had been made, a solenoid valve opened allowing 0.6 ml of liquid to flow from the 2000-ml reservoirs suspended above and mounted to the outside of the panel attached to the cage. Scheduling and recording of events were accomplished using Med-PC software (Med-PC® for Windows) and associated interfaces (Med Associates, St Albans, VT) located in an adjacent room.
2.3 Self-administration procedure
Monkeys self-administered PCP (0.25 mg/ml) and water according to concurrent FR 16 schedules of reinforcement during daily 3-h sessions. Experimental sessions were conducted 7 days per week from 1000 h to 1300 h. Completion of FR contingencies for lip contacts on the two spouts were independent of each other such that completion of the FR on one spout was unaffected by responses on the other spout. There was no timeout after completion of the FR and subsequent liquid delivery. PCP and water were available on alternating sides each day to avoid development of a side preference. Monkeys were initially tested with 0.25 mg/ml PCP, followed by tests with concentrations of 0.5 and 0.125 mg/ml. Each monkey self-administered each concentration of PCP for at least 5 cycles except for monkey M-M who completed only two cycles of self-administration of 0.125 mg/ml PCP.
2.4 Menstrual cycle phase verification
Menstrual cycle phase was confirmed by observation of menses onset and examination of vaginal cytology. Monkeys were checked daily for menstrual bleeding and vaginal swabbing was performed 1 h prior to the experimental session (0900 h) three times per week. Cytology of vaginal epithelium was classified according to the method described by Mauro et al (1970). Briefly, the vaginal wall was swabbed with a cotton-tipped applicator and the sample transferred to slides, stained with methylene blue, and cover slipped. Each phase was confirmed by the type of epithelial cells present. Menses was characterized by a predominance of parabasal and anucleated/cornified epithelial cell types, the follicular phase was characterized by the presence of superficial, intermediate, and anucleated/cornified cell types, and the luteal phase was characterized by the presence of parabasal, superficial, intermediate and annucleated/cornified epithelial cells.
2.5 Drugs
Phencyclidine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). PCP solutions were mixed in tap water 24 h prior to each session, and they were stored at room temperature. The PCP concentrations (0.125, 0.25, and 0.5 mg/ml) refer to the weight of the HCl salt.
2.6 Data analysis
Data obtained during the follicular phase, defined as days 3–10 following onset of menses, and from the luteal phase, defined as the last 8 days of a cycle preceding an onset of menses were used for analyses. PCP self-administration was further examined during early and mid-follicular phases, days 3–6 and 7–10, respectively, following menses onset, and during mid-luteal (the first 4 days of the 8-day luteal cycle) and late luteal (the last 4 days of the 8-day luteal cycle) phases. Number of PCP and water deliveries and PCP intake (mg/kg) served as dependent variables. The mean numbers of PCP and water deliveries were analyzed using a three-way (concentration × cycle phase × liquid) repeated measures analysis of variance (ANOVA). Mean PCP intake obtained during the early and mid-follicular and mid- and late luteal phases were compared separately using a one-way repeated measures ANOVA for each concentration. Newman-Keuls post-hoc tests were conducted when overall main effects were found to be significant (P < .05).
3. Results
3.1 Effect of PCP self-administration on menstrual cycle duration
The menstrual cycles in the three monkeys remained consistent across the study, although the length of cycle slightly increased for two monkeys when the highest concentration, 0.5 mg/ml, was self-administered. When 0.125 mg/ml PCP was self-administered, the range of days for menstrual cycle was 27–29 days for monkey M-M; 23–28 days for M-L; 22–28 days for M-P. For the concentration of 0.25 mg/ml PCP the range of days for the menstrual cycle was 25–29 days for monkey M-M; 23–28 days for M-L; and 23–28 days for M-P. When 0.5 mg/ml PCP was self-administered, number of days of menstrual cycles ranged from 23–32 for monkey M-M; 25–38 days for M-L, and 23–29 days for M-P.
3.2 PCP self-administration during follicular and luteal phases
Mean numbers of PCP and water deliveries are presented in Figure 1 as a function of cycle phase and PCP concentration. A significant main effect was found for phase [F(1, 1775) = 41.37, P < .0001]. Significantly greater numbers of deliveries were obtained during the luteal phase compared to the follicular phase when 0.125 mg/ml PCP (P < .01) and 0.25 mg/ml PCP (P < .01) were available. A significant main effect was also found across concentrations of PCP within each phase [F(2, 1775) = 30.40; P < .0001]. Fewer deliveries were obtained when 0.5 mg/ml PCP was available compared to 0.125 mg/ml (P < .01) and 0.25 mg/ml (P < .01) and this occurred during both phases. The number of PCP deliveries self-administered was significantly higher than that of water deliveries [F(1, 1775) = 645.58, P < .0001]. Post-hoc comparisons revealed that across all concentrations and for both phases, the numbers of PCP deliveries obtained were significantly greater than the number of water deliveries (P < .01 at all concurrent concentrations). There was no significant difference in numbers of water deliveries as a function of cycle phase; however, there were significant differences in water deliveries as a function of concurrent PCP concentration [F(2, 1775) = 23.05, P < .0001]. The number of water deliveries obtained when the concurrent PCP concentration was 0.25 mg/ml was significantly higher than water deliveries obtained when 0.125 (P < .01) or 0.5 (P < .01) mg/ml were concurrently available under both phase conditions.
Fig 1.
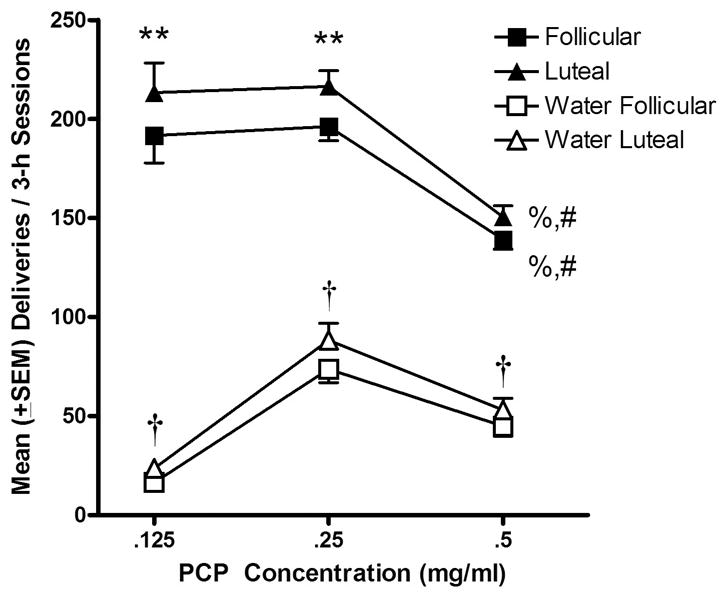
Mean (± SEM) numbers of PCP (filled symbols) and water (empty symbols) deliveries obtained during follicular phase (days 3–10 following onset of menses; squares) and luteal phase (final 8 days of each cycle; triangles) as a function of PCP concentration. ** P < .01 compared to deliveries obtained during the follicular phase. % P < .01 compared to 0.125 mg/ml within each phase; # P < .01 compared to 0.25 mg/ml within each phase; †P < .01 compared to PCP during both phases.
Numbers of PCP deliveries obtained during the follicular and luteal phases for each of the three subjects are shown in Table 1. For monkeys M-P and M-L, 0.25 mg/ml PCP maintained greater numbers of deliveries than either 0.125 or 0.5 mg/ml PCP. For monkey M-M, however, 0.125 mg/ml PCP maintained the greatest numbers of deliveries. The highest concentration of PCP, 0.5 mg/ml, maintained the fewest deliveries for monkeys M-M and M-L.
Table 1.
Mean numbers of PCP deliveries across multiple cycles and concentrations of PCP for individual monkeys.
| PCP 0.125 mg/ml | PCP 0.25 mg/ml | PCP 0.5 mg/ml | ||||
|---|---|---|---|---|---|---|
| Monkey | Follicular | Luteal | Follicular | Luteal | Follicular | Luteal |
|
| ||||||
| M-M | 447.30 | 483.80 | 159.50 | 170.50 | 116.30 | 99.67 |
| M-L | 235.60 | 256.00 | 274.30 | 312.90 | 191.50 | 214.30 |
| M-P | 86.27 | 105.60 | 154.70 | 168.30 | 97.94 | 117.50 |
3.3 PCP self-administration during early, mid-follicular, mid-, and late luteal phases
Mean (±SEM) numbers of PCP deliveries as a function of menstrual cycle phase for each of three concentrations is shown in Figure 2. At the lowest concentration, 0.125 mg/ml, numbers of PCP deliveries obtained differed as a function of phase [F(3, 239) = 3.83, P < .05]. Post-hoc tests indicated numbers of deliveries during the mid-luteal phase were significantly greater during than those obtained during early and mid-follicular (Ps < .05) and late luteal phases (P < .05). For 0.25 mg/ml, PCP deliveries differed across the four phases [F(3, 383) = 3.95, P < .05]. Post-hoc tests revealed significantly greater numbers of PCP deliveries were obtained during the mid-luteal phase compared to the mid-follicular phase (P < .01), and during late luteal compared to mid-follicular phases (P < .05). For the highest concentration of PCP tested, 0.5 mg/ml, a significant difference was also found across phases [F(3, 287) = 3.27, P < .05]. Post-hoc tests indicated that greater numbers of deliveries were obtained during the mid-luteal compared to the mid-follicular phase (P < .05).
Fig 2.
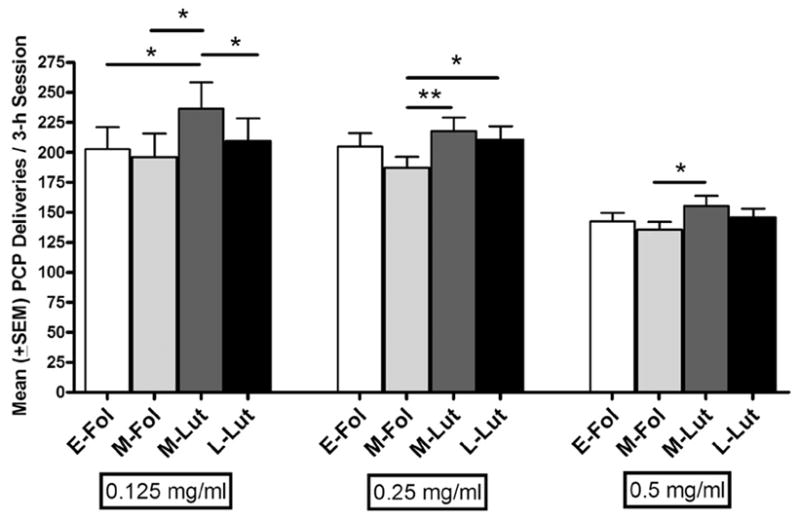
Mean (± SEM) numbers of PCP deliveries during early follicular (days 3–6), mid-follicular (7–10), mid-luteal (first 4 of final 8 days of each cycle) and late luteal (final 4 days of each cycle) phases. Concentrations of PCP available for self-administration are shown below each set of bars. *P < .05, **P < .01.
3.4 PCP intake during early, mid-follicular, mid-, and late luteal phases
Figure 3 shows mean (± SEM) PCP intake (mg/kg) obtained across 28 days of several menstrual cycles for the three monkeys when 0.25 mg/ml was the available concentration of PCP. An overall increase in intake was seen as the cycle progressed. Greater variability observed toward the end of the cycle and incongruity with numbers of deliveries during the late luteal phase is due to a different number of observations per monkey, as not all monkeys experienced 28-day menstrual cycles. A one-way repeated measures ANOVA comparing the mean intake of PCP during the early follicular (days 3–6), mid-follicular (days 7–10), mid-luteal (days 21–24), and late luteal (days 25–28) phases revealed a significant overall effect [F(3, 15) = 10.45, P < .01]. Post-hoc analyses revealed significant differences in intake between early follicular and late luteal (P < .05), mid-follicular and late luteal (P < .01), and mid-follicular and mid-luteal (P < .05) phases.
Fig 3.
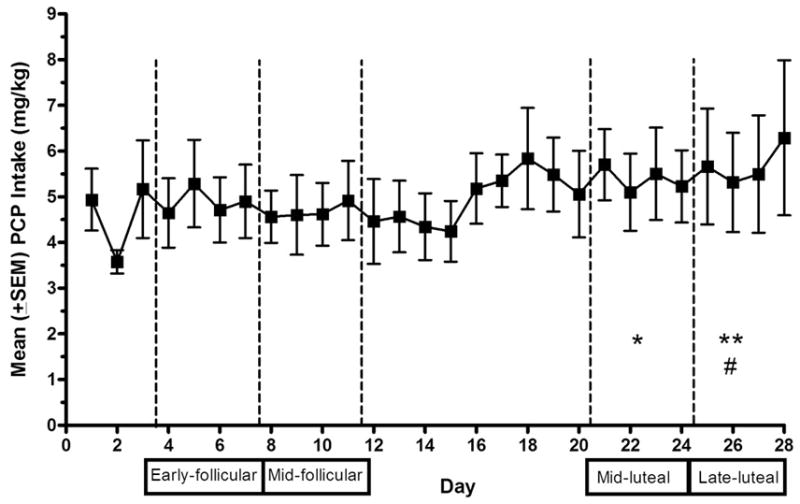
Mean (± SEM) intake of PCP (mg/kg) across several 28-day cycles when 0.25 mg/ml was available for self-administration. Early follicular (days 3–6 following menses onset), mid-follicular (days 7–10), mid-luteal (days 21–24) and late luteal (days 25–28) phases are separated by vertical dashed lines. *P < .05, **P < .01 compared to the mid-follicular phase; # P < .05 compared to the early follicular phase.
4. Discussion
The results from the present study indicate greater levels of PCP were self-administered during the luteal phase of the menstrual cycle. Further examination of the data within each phase revealed that the greatest numbers of PCP deliveries were obtained within the mid-luteal phase across all three concentrations of PCP. Self-administration of PCP was comparable between the early and mid-follicular phases, and between the mid- and late luteal phases, although a significantly greater number of PCP deliveries was obtained during the mid-luteal phase when the 0.125 mg/ml concentration was available. Intake of PCP also increased as a function of menstrual cycle when 0.25 mg/ml PCP was the available concentration.
The difference in PCP self-administration across menstrual cycle phase could be due to fluctuation in levels of estrogen and progesterone. As the mid-luteal phase is usually identified by a surge of plasma progesterone levels, the difference may be attributable to higher progesterone levels. As plasma hormone levels were not evaluated in these monkeys, this conclusion remains speculative. The finding that PCP was self-administered at greater levels during the luteal phase in the present study is contrary to the reports from other studies on the subjective effects produced by psychostimulants across the menstrual cycle. However, the present study is distinct from most others in that the drug was self-administered rather than being administered noncontingently by the experimenter.
The shift in the concentration-response curves between follicular and luteal phases could represent either an increase in the reinforcing effects of PCP during the luteal phase or an increase in self-administration as a compensatory attempt to overcome negative or inhibitory effects that may be associated with increases in progesterone. It has been suggested that progesterone may reduce the positive effects associated with drugs. For example, Sofuoglu (2004) found that women reported decreases on ‘feel the effects of the last dose’, but did not decrease the number of cocaine choices compared to the placebo treatment. Both Sofouglu et al. (2002 Both Sofouglu et al. (2001) and Evans and Foltin (2006a) reported that, following treatment with progesterone during the follicular phase, females reported diminished positive subject-rated effects of cocaine. In another study conducted by Evans et al (2002) cocaine improved some of the negative mood states present during the luteal phase in women with no histories of premenstrual dysphoric disorders.
Another difference between the present and previous studies is the pharmacological class of the drug being examined. PCP, like cocaine, blocks dopamine reuptake (Di Chiara and Imperato, 1988; Gerhardt et al., 1987). However, PCP inhibits glutamate transmission by blocking the NMDA receptor channel, an action that is also involved in its reinforcing effects (Carlezon and Wise, 1996). While estrogen and progesterone affect dopamine neurotransmission in brain areas related to the reinforcing effects of drugs (Becker, 1999; Shimizu and Bray, 1993; Thompson and Moss, 1994, 1997), they also affect neurotransmission mediated by the NMDA receptor. Exogenously administered progesterone and estrogen, or estrogen alone, produces decreases in NMDA receptor binding density in the frontal cortex of rats (Cyr et al., 2000; 2001). The contribution of genomic and non-genomic effects of the steroid hormones with regard to NMDA receptors (McEwen, 2002, 1996) adds to the complexity of potential interactions of estrogen and progesterone with the pharmacological effects of PCP related to its reinforcing effects. Furthermore, progesterone-derived neurosteroids, such as pregnanolone and allopregnanolone, are behaviorally active (Grant et al., 1997; Mathis et al., 1996) positive neuromodulators of NMDA receptors (Bowlby, 1993; Irwin et al., 1994; Wu et al., 1991). Allopregnanolone itself has been shown to modulate NMDA-stimulated dopamine release in a progesterone- and estrogen-dependent manner (Cabrera et al., 2002). Thus, the complex interaction between PCP’s action as an NMDA receptor antagonist and elevated levels of progesterone and its associated neurosteroids could play a role in the increased PCP self-administration during the luteal phase.
It is possible that PCP’s pharmacokinetics may differ as a result of menstrual cycle phase, and this could, in turn alter the sensitivity to its reinforcing effects. While it has been demonstrated that metabolism of PCP differs between male and female rats (Nabeshima et al., 1984; Shelnutt et al., 1999), the relative contribution of ovarian hormones to the metabolism of PCP has not been examined. Peak plasma levels of cocaine differ between the follicular and luteal phases in humans (Lukas et al., 1996). However, Mello et al. (1984) did not find differences in blood levels of ethanol, across the menstrual cycle in rhesus monkeys. Other studies have shown that in humans (Mendelson et al., 1999) and rhesus monkeys (Evans and Foltin, 2004; 2006b) pharmacokinetics of cocaine do not differ as a function of menstrual phase. Additionally, as the self-administration histories of these monkeys were extensive, the possibility of altered hormone release as a consequence should be considered. However, previous studies conducted by others (Ferin et al., 1976) have demonstrated that repeated treatment with sedative doses of PCP did not alter monkeys’ menstrual cycles. Similarly, daily sedation with ketamine, a drug that is pharmacologically similar to PCP, failed to affect menstrual cycle length, or serum progesterone and estrogen levels, and may have reduced the occurrence of anovulatory cycles (Channing et al., 1977).
Effects of steroid hormones that are not specific to drug-taking behaviors should also be taken into account. Consummatory behaviors may vary systematically as a function of menstrual cycle phase. For example, during the preovulatory period, food intake decreased in intact rhesus monkeys and in ovariectomized rhesus monkeys treated with estrogen, an effect that was reduced by co-administration of progesterone and not attributable to taste preferences (Kemnitz et al., 1989). Roth et al. (2005) did not observe systematic differences in lever pressing maintained by food presentation as a function of menstrual cycle phase in cynomolgus monkeys. In the present study, water intake did not change as a function of menstrual cycle phase suggesting the difference in oral PCP self-administration was not related to general increases in liquid consumption.
Most studies conducted to date have used serum gonadotropin and steroid hormone levels to verify menstrual cycle phase and occurrence of ovulation. In the present study, menstrual cycle phase was determined by onset of menses and verified by classification of vaginal cytology. Although the presence of certain cell types is indicative of phase-related fluctuations of circulating hormones across the menstrual cycle (Mauro et al., 1970; Mehta et al., 1986; Stute et al., 2004), quantification of serum progesterone and estrogen levels is the best determinant of circulating hormone levels and occurrence of ovulation. A second limitation of the current study is the small sample size used. Only three normally cycling female monkeys were available for this study; however, the experiment was conducted over an 18 month period and multiple cycles per monkey were used. Increasing the size of the sample would strengthen the ability to generalize the current findings to those of other studies.
Previous studies demonstrated that PCP self-administration differs between male and female rhesus monkeys. This is the first report on PCP’s reinforcing effects across the menstrual cycle. Results from the present study demonstrated that self-administration of PCP differed across menstrual cycle, implicating a role for endogenous hormones in its reinforcing effects. PCP self-administration was found to be greater during the luteal phase, and more specifically, the mid-luteal phase, when progesterone levels are normally high compared to the follicular phase when progesterone levels are normally low. Further research is necessary to evaluate the relative effects of estrogen and progesterone on PCP’s reinforcing effects, and to identify the pharmacological mechanisms involved in mediating the increase in PCP self-administration during the luteal phase.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants R01 DA002486-26 and K05 DA015267-04 (MEC) and National Institute on Drug Abuse training grant T32 DA07097 (JLN). The authors wish to thank Dr. Megan Roth and Alayna Fogal for their expert technical assistance, and Dr. Erin Larson and Justin Anker for their critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res. 2000;2:231–41. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. J Subst Abuse. 1996;8:303–19. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Carroll ME. College on Problems of Drug Dependence. Scottsdale, AZ: 2006. Effects of Progesterone and Estrogen on Reinstatement of Cocaine-Seeking Behavior in Female Rats. [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–12. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bowlby MR. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Mol Pharmacol. 1993;43:813–9. [PubMed] [Google Scholar]
- Cabrera RJ, Bregonzio C, Laconi M, Mampel A. Allopregnanolone increase in striatal N-methyl-D-aspartic acid evoked [3H]dopamine release is estrogen and progesterone dependent. Cell Mol Neurobiol. 2002;22:445–54. doi: 10.1023/A:1021015705597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996;128:413–20. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 2005;180:414–26. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Roth ME, Voeller RK, Nguyen PD. Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacology (Berl) 2000;149:401–8. doi: 10.1007/s002130000389. [DOI] [PubMed] [Google Scholar]
- Channing CP, Fowler S, Engel B, Vitek K. Failure of daily injections of ketamine HCL to adversely alter menstrual cycle length, blood estrogen, and progesterone levels in the rhesus monkey. Proc Soc Exp Biol Med. 1977;155:615–9. doi: 10.3181/00379727-155-39862. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Differential effects of bremazocine on oral phencyclidine (PCP) self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:111–7. doi: 10.1037/1064-1297.12.2.111. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology (Berl) 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12:445–52. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev. 2001;37:153–61. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006a;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of intravenous cocaine across the menstrual cycle in rhesus monkeys. Neuropsychopharmacology. 2004;29:1889–900. doi: 10.1038/sj.npp.1300486. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in rhesus monkeys. Pharmacol Biochem Behav. 2006b;83:56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Ferin M, Carmel PW, Warren MP, Himsworth RL, Frantz AG, Nocenti MR. Phencyclidine sedation as a technique for handling rhesus monkeys: effects on LH, GH, and prolactin secretion. Proc Soc Exp Biol Med. 1976;151:428–33. doi: 10.3181/00379727-151-39227. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien C, Childress A. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6:171–5. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Pang K, Rose GM. In vivo electrochemical demonstration of the presynaptic actions of phencyclidine in rat caudate nucleus. J Pharmacol Exp Ther. 1987;241:714–21. [PubMed] [Google Scholar]
- Goodman AL, Hodgen GD. The ovarian triad of the primate menstrual cycle. Recent Prog Horm Res. 1983;39:1–73. doi: 10.1016/b978-0-12-571139-5.50005-7. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3 alpha-hydroxy-5 alpha-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology (Berl) 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Mendelson JH, Mello NK, Lex BW. Marihuana use across the menstrual cycle. Drug Alcohol Depend. 1986;18:213–24. doi: 10.1016/0376-8716(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–45. [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology (Berl) 2000;150:374–82. doi: 10.1007/s002130000461. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studies. J Pharmacol Exp Ther. 1994;271:677–82. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000a;71:51–9. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm Behav. 1989;23:235–50. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Lex BW, Mendelson JH, Bavli S, Harvey K, Mello NK. Effects of acute marijuana smoking on pulse rate and mood states in women. Psychopharmacology (Berl) 1984;84:178–87. doi: 10.1007/BF00427443. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–54. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Marks JL, Pomerleau CS, Pomerleau OF. Effects of menstrual phase on reactivity to nicotine. Addict Behav. 1999;24:127–34. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Mathis C, Vogel E, Cagniard B, Criscuolo F, Ungerer A. The neurosteroid pregnenolone sulfate blocks deficits induced by a competitive NMDA antagonist in active avoidance and lever-press learning tasks in mice. Neuropharmacology. 1996;35:1057–64. doi: 10.1016/s0028-3908(96)00041-x. [DOI] [PubMed] [Google Scholar]
- Mauro J, Serrone D, Somsin P, Stein A. Cyclic Vaginal Cytologic Patterns in the Macaca Mulatta. Acta Cytologica. 1970;14:348–52. [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McEwen B. Gonadal and adrenal steroids regulate neurochemical and structural plasticity of the hippocampus via cellular mechanisms involving NMDA receptors. Cellular and Molecular Neurobiology. 1996;16:103–16. doi: 10.1007/BF02088170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RR, Jenco JM, Gaynor LV, Chatterton RT., Jr Relationships between ovarian morphology, vaginal cytology, serum progesterone, and urinary immunoreactive pregnanediol during the menstrual cycle of the cynomolgus monkey. Biol Reprod. 1986;35:981–6. doi: 10.1095/biolreprod35.4.981. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Skupny AS, Mendelson JH. Blood alcohol levels as a function of menstrual cycle phase in female macaque monkeys. Alcohol. 1984:1. doi: 10.1016/0741-8329(84)90032-6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Knudson IM, Kelly M. College on Problems of Drug Dependence. Orlando, FL USA: 2005. Acute effects of testosterone and progesterone on cocaine self-administration by female rhesus monkeys. [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T. Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur J Pharmacol. 1984;97:217–27. doi: 10.1016/0014-2999(84)90453-9. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Wahlstrom G, Backstrom T, Sundstrom Poromaa I. Altered sensitivity to alcohol in the late luteal phase among patients with premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29:767–77. doi: 10.1016/S0306-4530(03)00121-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, Keins A, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol. 2000;68:176–80. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Perry JL, Normile LM, Morgan AD, Carroll ME. Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys (Macaca mulatta) Exp Clin Psychopharmacol. 2006;14:68–78. doi: 10.1037/1064-1297.14.1.68. [DOI] [PubMed] [Google Scholar]
- Pohl CR, Knobil E. The role of the central nervous system function in the control of ovarian function in higher primates. Annual Reviews in Physiology. 1982;44:583–93. doi: 10.1146/annurev.ph.44.030182.003055. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. J Subst Abuse. 1994;6:227–34. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–46. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Roth ME, Negus SS, Knudson IM, Burgess MP, Mello NK. Effects of gender and menstrual cycle phase on food-maintained responding under a progressive-ratio schedule in cynomolgus monkeys. Pharmacol Biochem Behav. 2005;82:735–43. doi: 10.1016/j.pbb.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelnutt SR, Gunnell M, Owens SM. Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J Pharmacol Exp Ther. 1999;290:1292–8. [PubMed] [Google Scholar]
- Shimizu H, Bray GA. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res. 1993;621:200–6. doi: 10.1016/0006-8993(93)90107-x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Stute P, Wood CE, Kaplan JR, Cline JM. Cyclic changes in the mammary gland of cynomolgus macaques. Fertil Steril. 2004;82 (Suppl 3):1160–70. doi: 10.1016/j.fertnstert.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229:145–8. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–6. [PubMed] [Google Scholar]


