Abstract
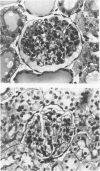
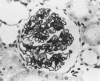
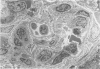
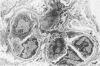
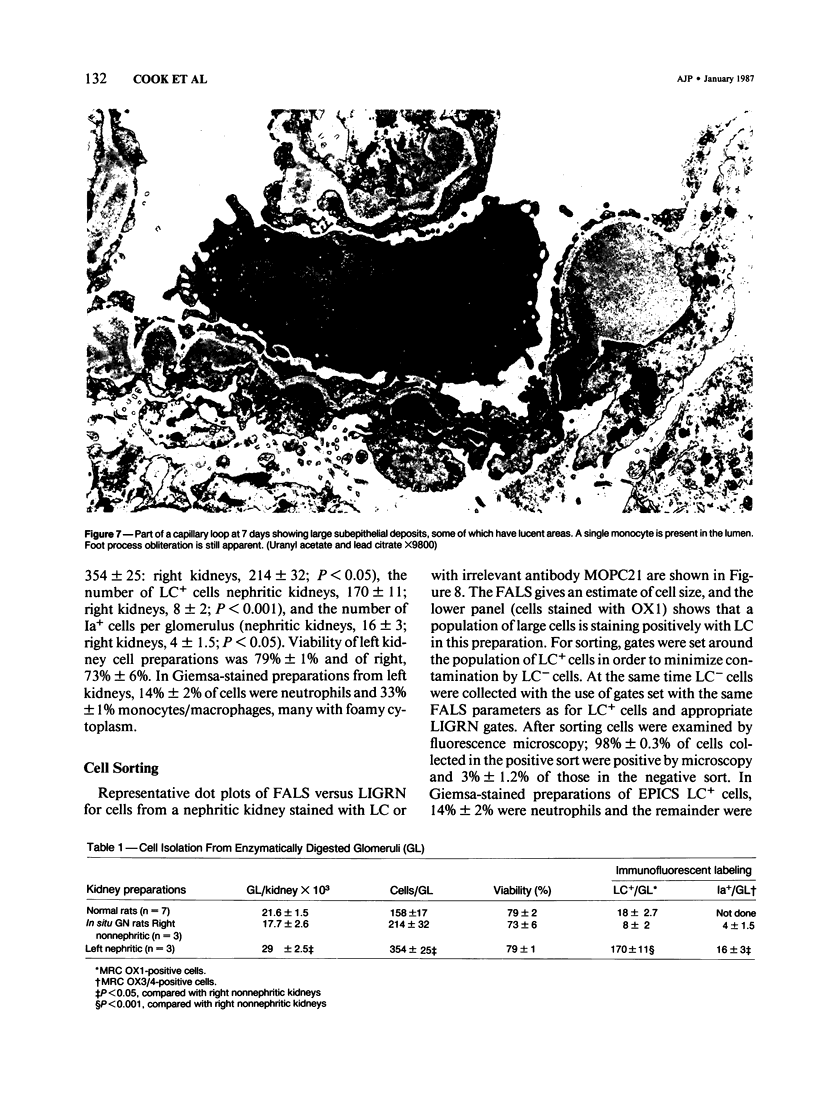
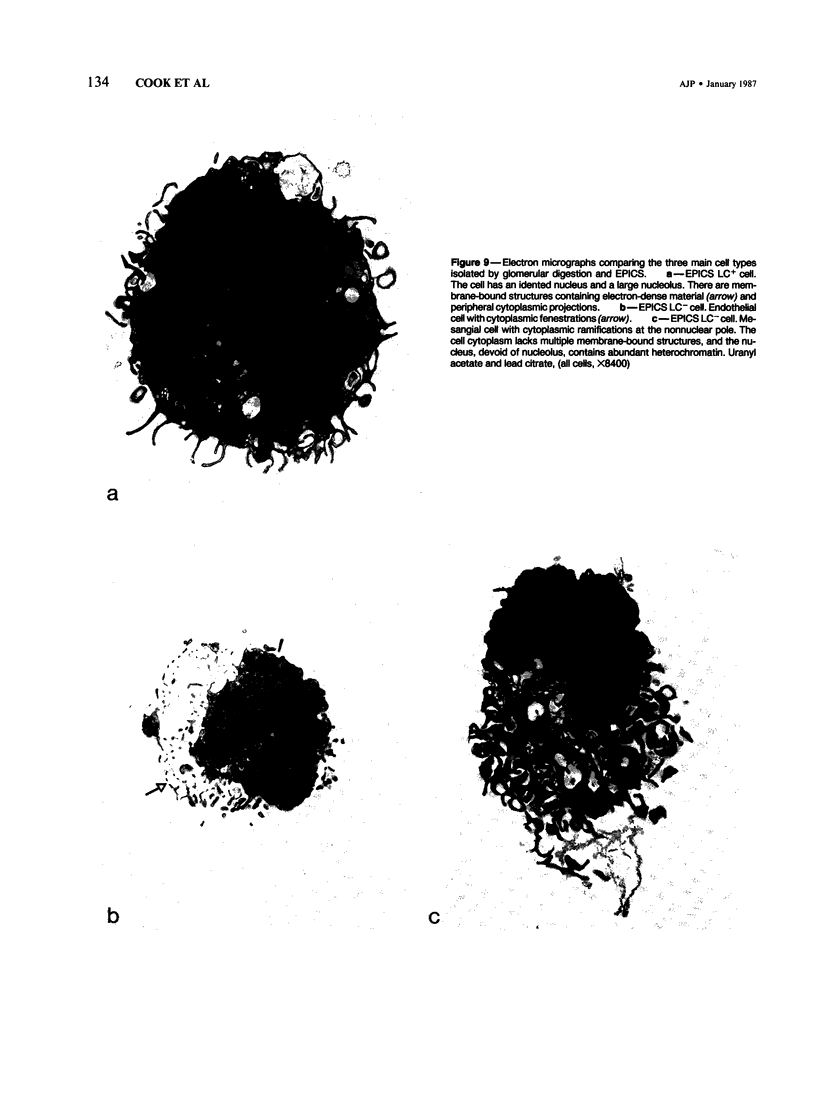
Inflammatory cell populations in glomerulonephritis (GN) are not well characterized. A method is reported for isolating leukocytes from glomeruli. GN was induced in rats by perfusing left kidneys (LKs) with cationized human IgG followed by intravenous rat anti-human IgG serum. Acute GN developed in LKs with proteinuria, deposition of human and rat IgG and C3, leukocyte infiltration, and capillary wall electron-dense deposits. Glomeruli (GL) isolated at 24 hours were digested with collagenase, trypsin, and DNase, and the resulting cells were as follows (mean +/- SEM): LK, 354 +/- 25/GL; RK, 214 +/- 32/GL. Cells were labeled with monoclonal antibody MRCOX1 (anti-rat leukocyte common [LC] antigen) followed by FITC F(ab')2 rabbit anti-mouse Ig: LK, 170 +/- 11 leukocytes/GL;RK, 8 +/- 2 leukocytes/GL (P less than 0.001). Isolated cells were sorted by flow cytometry to 98% pure LC+ cells with greater than 80% viability (Giemsa staining: 86% mononuclear cells, 14% neutrophils); the ultrastructure was that of maturing macrophages and neutrophils. This method quantitates leukocyte infiltration and provides leukocytes from nephritic glomeruli suitable for in vitro studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. II. The effect of Mycobacterium tuberculosis. Am J Pathol. 1975 Jul;80(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Agodoa L. Y., Gauthier V. J., Mannik M. Precipitating antigen-antibody systems are required for the formation of subepithelial electron-dense immune deposits in rat glomeruli. J Exp Med. 1983 Oct 1;158(4):1259–1271. doi: 10.1084/jem.158.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins R. C., Glasgow E. F., Holdsworth S. R., Thomson N. M., Hancock W. W. Tissue culture of isolated glomeruli from patients with glomerulonephritis. Kidney Int. 1980 Apr;17(4):515–527. doi: 10.1038/ki.1980.60. [DOI] [PubMed] [Google Scholar]
- Camazine S. M., Ryan G. B., Unanue E. R., Karnovsky M. J. Isolation of phagocytic cells from the rat renal glomerulus. Lab Invest. 1976 Oct;35(4):315–326. [PubMed] [Google Scholar]
- Cattell V., Gaskin de Urdaneta A., Arlidge S., Collar J. E., Roberts A., Smith J. Uptake and clearance of ferritin by the glomerular mesangium. I. Phagocytosis by mesangial cells and blood monocytes. Lab Invest. 1982 Sep;47(3):296–303. [PubMed] [Google Scholar]
- Cohn Z. A., Hirsch J. G., Fedorko M. E. The in vitro differentiation of mononuclear phagocytes. IV. The ultrastructure of macrophage differentiation in the peritoneal cavity and in culture. J Exp Med. 1966 Apr 1;123(4):747–756. doi: 10.1084/jem.123.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook H. T., Cattell V., Smith J., Salmon J. A., Moncada S. Effect of a thromboxane synthetase inhibitor on eicosanoid synthesis and glomerular injury during acute unilateral glomerulonephritis in the rat. Clin Nephrol. 1986 Oct;26(4):195–202. [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Laohapand T., Smith J., Cattell V. Blood leucocyte infiltration after intravenous injection of ferritin in the rat. Br J Exp Pathol. 1985 Aug;66(4):475–482. [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Oite T., Batsford S. R., Mihatsch M. J., Takamiya H., Vogt A. Quantitative studies of in situ immune complex glomerulonephritis in the rat induced by planted, cationized antigen. J Exp Med. 1982 Feb 1;155(2):460–474. doi: 10.1084/jem.155.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G., Anversa P., Melissari M., Loud A. V. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980 Apr;17(4):438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- Salant D. J., Adler S., Darby C., Capparell N. J., Groggel G. C., Feintzeig I. D., Rennke H. G., Dittmer J. E. Influence of antigen distribution on the mediation of immunological glomerular injury. Kidney Int. 1985 Jun;27(6):938–950. doi: 10.1038/ki.1985.102. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Kiely J. M., Cotran R. S., Unanue E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981 Oct;68(4):920–931. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Origin of the rat mesangial phagocyte and its expression of the leukocyte common antigen. Lab Invest. 1984 Nov;51(5):515–523. [PubMed] [Google Scholar]
- Sinclair R. A., Burns J., Dunnill M. S. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a comparison of the peroxidase-antiperoxidase (PAP) and indirect methods. J Clin Pathol. 1981 Aug;34(8):859–865. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Vogt A., Schmidt H. U., Takamiya H., Batsford S. 'In situ' immune complex nephritis and basic proteins. Proc Eur Dial Transplant Assoc. 1980;17:613–620. [PubMed] [Google Scholar]