Abstract
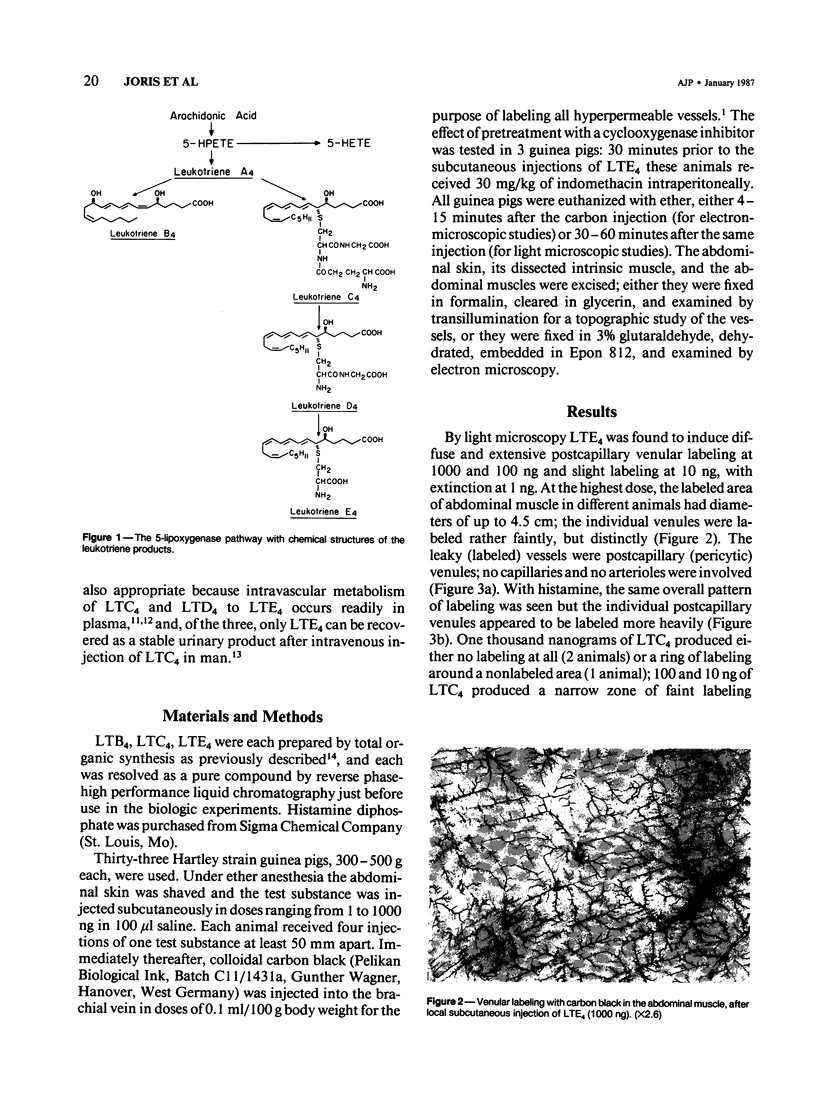
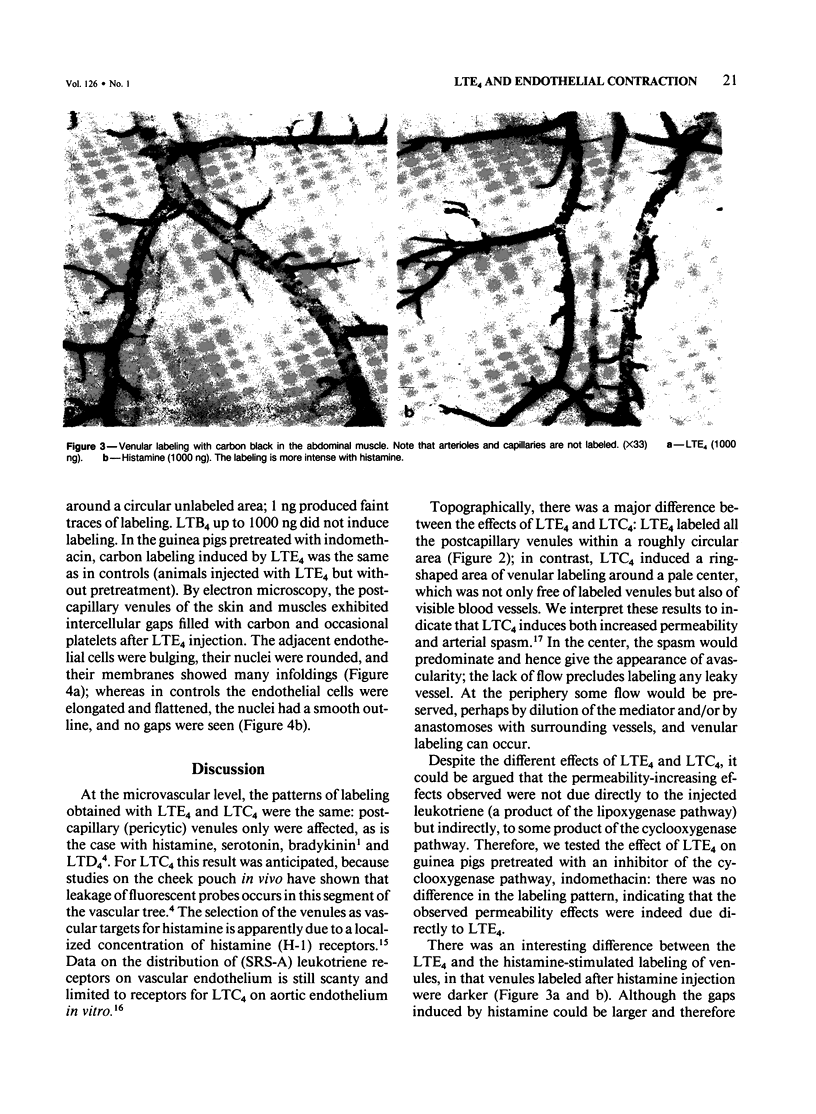
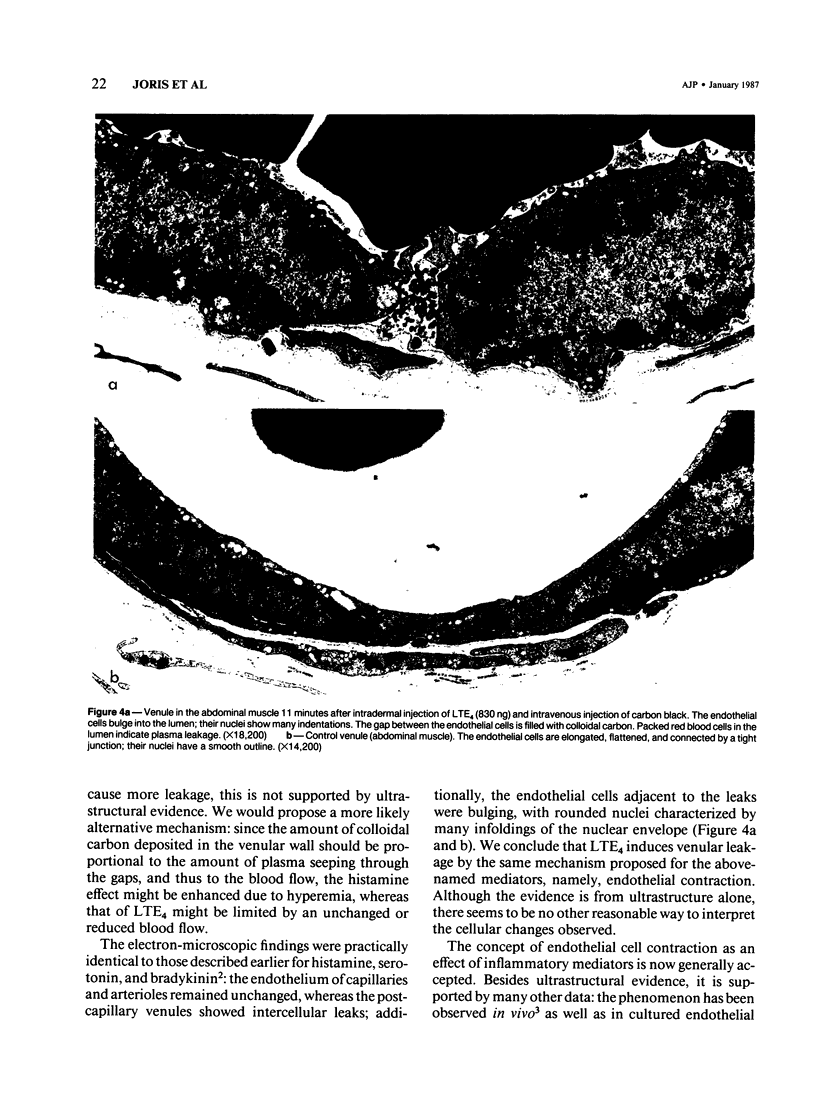
This study identifies the microvascular target of leukotriene E4 (LTE4) by vascular labeling with carbon black and establishes the mechanism of its action at the cellular level by electron microscopy. LTE4 and its tripeptide precursor, leukotriene C4 (LTC4) were injected subcutaneously in guinea pigs. With LTE4, venular labeling was intense at 1000 and 100 ng and slight at 10 ng, with extinction at 1 ng. LTC4 induced a ring of labeled venules around a blank central area, suggestive of vasospasm. The nonpeptidyl leukotriene LTB4 induced no labeling. Histamine (1000 ng) induced an area of vascular labeling about equal to that by 1000 ng LTE4, but the labeling of individual venules was more intense. By electron microscopy, LTE4 was found to induce gaps in the endothelium of the venules; the endothelial cells adjacent to the gaps bulged into the lumen and showed wrinkled nuclei, consistent with cellular contraction. This ultrastructural evidence suggests that LTE4 increases vascular permeability by contraction of endothelial cells selectively, in the postcapillary venules, as was previously demonstrated for other inflammatory mediators, including histamine, serotonin, and bradykinin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTRAN R. S. THE DELAYED AND PROLONGED VASCULAR LEAKAGE IN INFLAMMATION. II. AN ELECTRON MICROSCOPIC STUDY OF THE VASCULAR RESPONSE AFTER THERMAL INJURY. Am J Pathol. 1965 Apr;46:589–620. [PMC free article] [PubMed] [Google Scholar]
- Corey E. J. Chemical studies on slow reacting substances/leukotrienes. Experientia. 1982 Nov 15;38(11):1259–1275. doi: 10.1007/BF01954913. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck F., De Brabander M., Neels H., Van de Velde V. Direct evidence for the contractile capacity of endothelial cells. Thromb Res. 1981 Sep 15;23(6):505–520. doi: 10.1016/0049-3848(81)90174-2. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F., Lewis R. A., Clark D. A., Goto G., Marfat A., Corey E. J. Comparative airway and vascular activities of leukotrienes C-1 and D in vivo and in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4354–4358. doi: 10.1073/pnas.77.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Galey F., Wayland H. Action of histamine on the mesenteric microvasculature. Microvasc Res. 1980 Jan;19(1):108–126. doi: 10.1016/0026-2862(80)90087-4. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C. Early changes of endothelial clefts after thermal injury. Microvasc Res. 1975 Jul;10(1):65–75. doi: 10.1016/0026-2862(75)90020-5. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C., Mathewson S. M., Ryan G. B. Acute cadmium intoxication. Early selective lesions of endothelial clefts. Lab Invest. 1974 Jun;30(6):686–695. [PubMed] [Google Scholar]
- Hammersen F. Endothelial contractility - an undecided problem in vascular research. Beitr Pathol. 1976 May;157(4):327–348. doi: 10.1016/s0005-8165(76)80049-2. [DOI] [PubMed] [Google Scholar]
- Hammersen F. Endothelial filaments and intercellular gaps--a sufficient evidence for contractility? Bibl Anat. 1973;12:159–164. [PubMed] [Google Scholar]
- Heltianu C., Simionescu M., Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982 May;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan K. L., Adamski S. W., Ayele W., Langone J. J., Grega G. J. Evidence that prolonged histamine suffusions produce transient increases in vascular permeability subsequent to the formation of venular macromolecular leakage sites. Proof of the Majno-Palade hypothesis. Am J Pathol. 1986 Jun;123(3):570–576. [PMC free article] [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Hanahan D. J., Pinckard R. N. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984 Jan;50(1):16–25. [PubMed] [Google Scholar]
- Joris I., Majno G., Ryan G. B. Endothelial contraction in vivo: a study of the rat mesentery. Virchows Arch B Cell Pathol. 1972;12(1):73–83. doi: 10.1007/BF02893987. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., DeCamilli P., Boyles J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvasc Res. 1984 Sep;28(2):206–219. doi: 10.1016/0026-2862(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985 May;100(5):1379–1386. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol. 1985 May;100(5):1387–1395. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Austen K. F., Clark D. A., Corey E. J. Identification of the C(6)-S-conjugate of leukotriene A with cysteine as a naturally occurring slow reacting substance of anaphylaxis (SRS-A). Importance of the 11-cis-geometry for biological activity. Biochem Biophys Res Commun. 1980 Sep 16;96(1):271–277. doi: 10.1016/0006-291x(80)91210-3. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Figueiredo J. C., Corey E. J., Austen K. F. A review of recent contributions on biologically active products of arachidonate conversion. Int J Immunopharmacol. 1982;4(2):85–90. doi: 10.1016/0192-0561(82)90055-8. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning L., Kaijser L., Hammarström S. In vivo metabolism of leukotriene C4 in man: urinary excretion of leukotriene E4. Biochem Biophys Res Commun. 1985 Jul 16;130(1):214–220. doi: 10.1016/0006-291x(85)90404-8. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Koch D., Huber M. M., Falkenhein S. F. Formation of the cysteinyl form of slow reacting substance (leukotriene E4) in human plasma. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1038–1046. doi: 10.1016/0006-291x(80)91480-1. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Soter N. A., Lewis R. A., Corey E. J., Austen K. F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983 Feb;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Kilo C., Williamson J. R., Murch D. W. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res. 1979 Nov;18(3):336–352. doi: 10.1016/0026-2862(79)90042-6. [DOI] [PubMed] [Google Scholar]
- Woodward D. F., Weichman B. M., Gill C. A., Wasserman M. A. The effect of synthetic leukotrienes on tracheal microvascular permeability. Prostaglandins. 1983 Jan;25(1):131–142. doi: 10.1016/0090-6980(83)90142-9. [DOI] [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D. The effect of ethchlorvynol on cultured endothelial cells. A model for the study of the mechanism of increased vascular permeability. Am J Pathol. 1985 Jun;119(3):505–512. [PMC free article] [PubMed] [Google Scholar]