Abstract
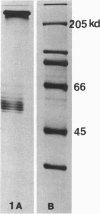
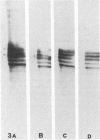
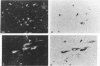
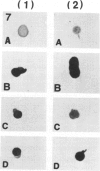
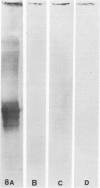
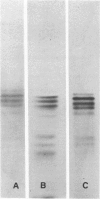
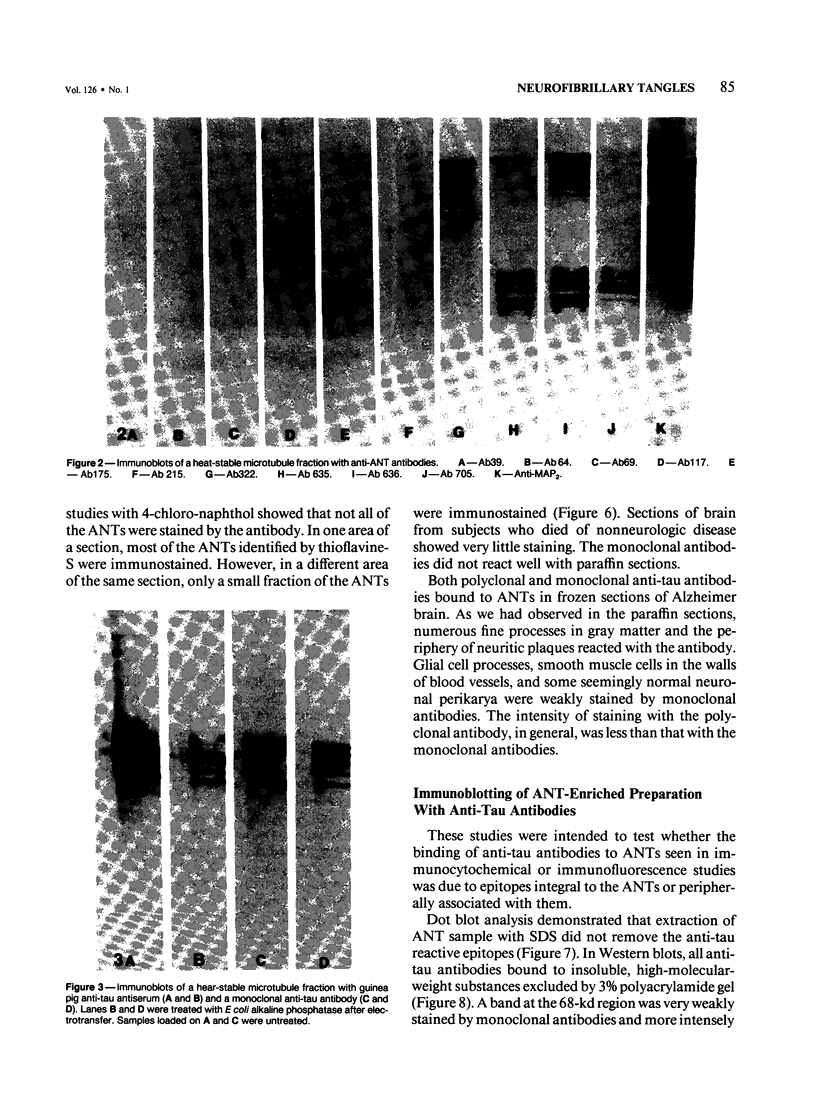
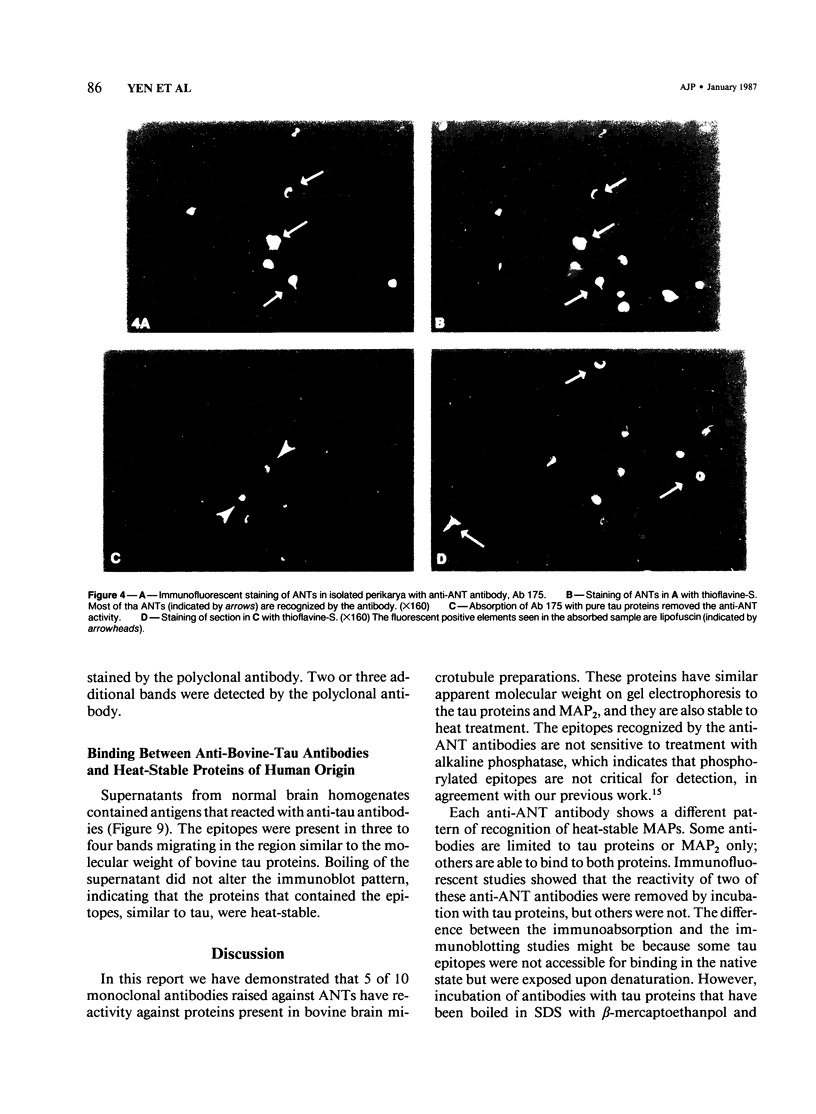
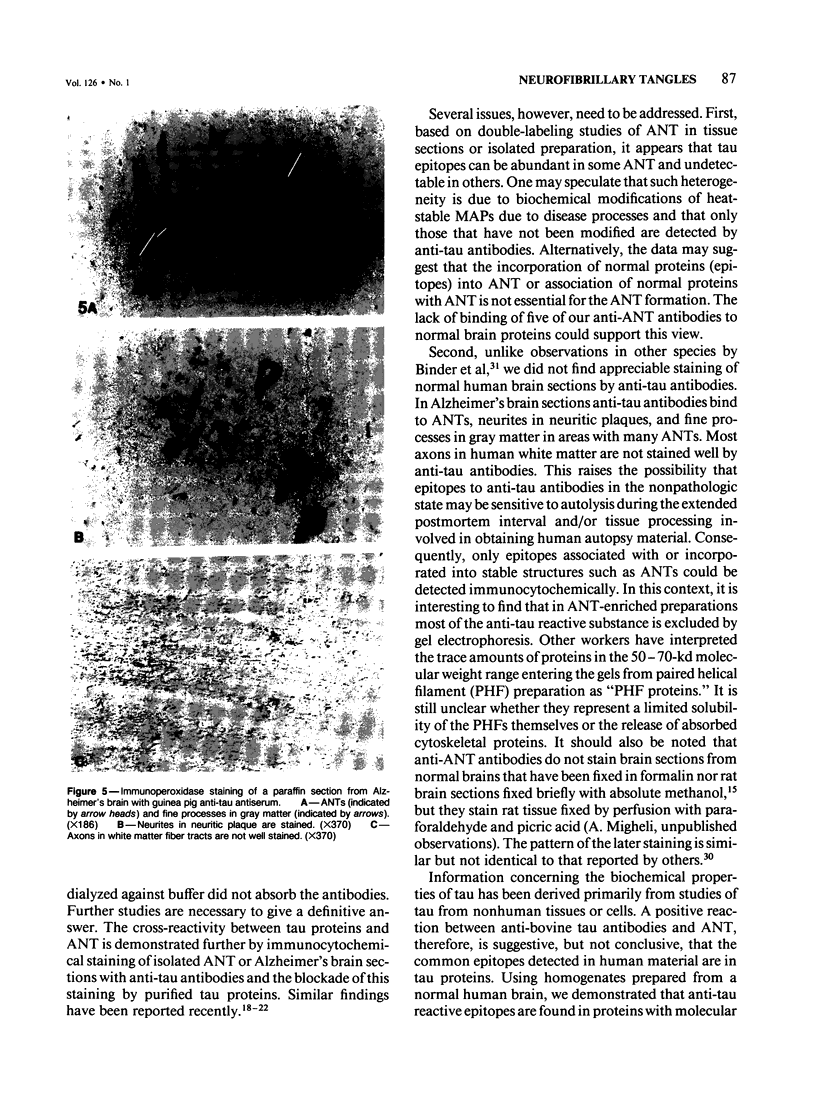
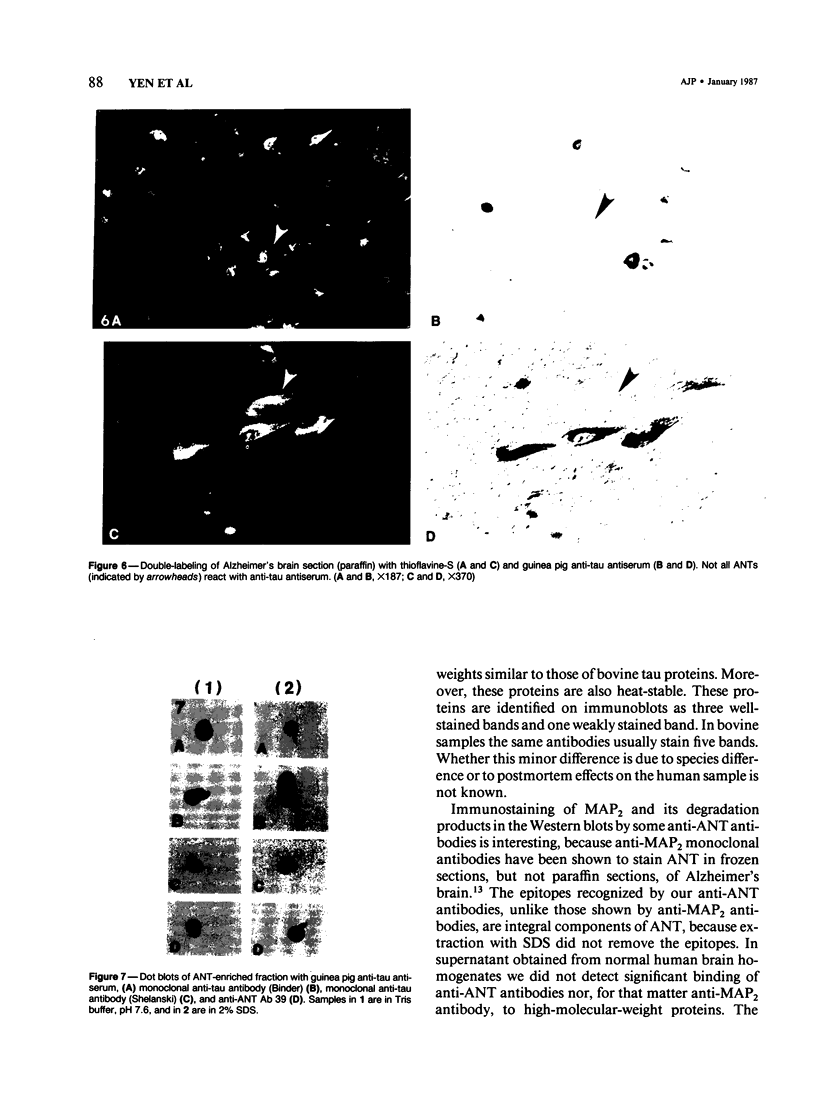
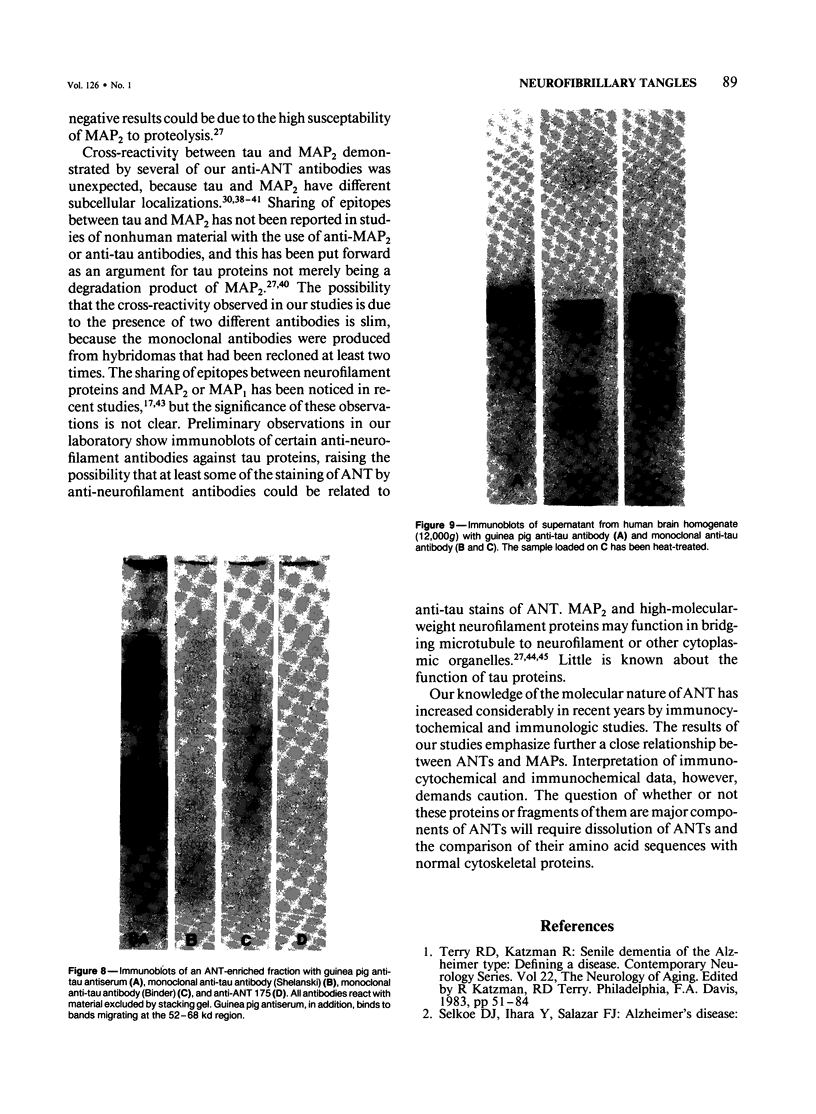
Ten monoclonal antibodies raised against Alzheimer's neurofibrillary tangles (ANTs) were characterized for reactivity with heat-stable microtubule fractions from bovine and human brain. Five of the antibodies showed very little reaction, but the other five reacted strongly with heat-stable microtubule associated proteins (MAPs). The proteins recognized by these antibodies have estimated molecular weights similar to those of known heat-stable MAPs, tau (52-68 kd) and MAP2 (200-250 kd). That the proteins are indeed tau and MAP2 is demonstrated by reaction of electroblotted proteins with antibodies raised in mouse and guinea pig against bovine brain tau and MAP2. One anti-ANT antibody reacts only with tau, two bind strongly to tau and weakly to MAP2, one recognizes both tau and MAP2 equally well, and one primarily stains MAP2. Extraction of ANT with 2% SDS does not remove tau or MAP2 epitopes from ANT, indicating that epitopes shared with heat-stable MAPs are integral components of ANT. The existence of tau epitopes in ANT is also demonstrated by immunoblotting of ANT-enriched fractions with anti-tau antibodies. Most of the material recognized by anti-tau antibodies in ANT-enriched fractions is present in large molecules excluded by 3% polyacrylamide gel upon electrophoresis. Anti-tau antibodies immunostain ANT in immunofluorescence and immunoperoxidase studies. The immunostaining can be blocked by absorption of anti-tau antibodies with purified tau proteins from bovine brain. Not all ANTs in any given tissue section or isolated Alzheimer perikarial preparations, however, are stained by anti-tau antibodies. These results are consistent with previous studies that have demonstrated heterogeneity of ANTs. Whether this heterogeneity is due to biochemical modification of MAPs or absence of MAPs in some ANTs is unknown. The significance of what appear to be shared epitopes recognized by monoclonal antibodies in tau and MAP2, and the implications this may have on the pathogenesis of ANT formation, requires further investigation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Bignami A., Selkoe D. J., Dahl D. Amyloid-like (Congophilic) neurofibrillary tangles do not react with neurofilament antisera in Alzheimer's cerebral cortex. Acta Neuropathol. 1984;64(3):243–250. doi: 10.1007/BF00688115. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M., Shelanski M. L. Microheterogeneity of microtubule-associated tau proteins is due to differences in phosphorylation. J Neurochem. 1986 Nov;47(5):1517–1522. doi: 10.1111/j.1471-4159.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Caceres A., Binder L. I., Payne M. R., Bender P., Rebhun L., Steward O. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1984 Feb;4(2):394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Miller P. E., Navone F., Theurkauf W. E., Vallee R. B. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984 Apr;11(4):817–846. [PubMed] [Google Scholar]
- Gambetti P., Shecket G., Ghetti B., Hirano A., Dahl D. Neurofibrillary changes in human brain. An immunocytochemical study with a neurofilament antiserum. J Neuropathol Exp Neurol. 1983 Jan;42(1):69–79. doi: 10.1097/00005072-198301000-00006. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Johnson A. B., Wisniewski H. M., Terry R. D., Iqbal K. Evidence that Alzheimer neurofibrillary tangles originate from neurotubules. Lancet. 1979 Mar 17;1(8116):578–580. doi: 10.1016/s0140-6736(79)91006-7. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Zaidi T., Thompson C. H., Merz P. A., Wisniewski H. M. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62(3):167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984 Oct 10;259(19):12241–12245. [PubMed] [Google Scholar]
- McKeithan T. W., Rosenbaum J. L. The biochemistry of microtubules. A review. Cell Muscle Motil. 1984;5:255–288. doi: 10.1007/978-1-4684-4592-3_7. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Aamodt E. J. Interactions between microtubules and neurofilaments in vitro. Ann N Y Acad Sci. 1985;455:509–524. doi: 10.1111/j.1749-6632.1985.tb50432.x. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Crowe A., Dickson D. W. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 1. Identification of polypeptides. Am J Pathol. 1985 Aug;120(2):282–291. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Fu S. M. Neurofibrillary tangles in senile dementia of the Alzheimer type share an antigenic determinant with intermediate filaments of the vimentin class. Am J Pathol. 1983 Dec;113(3):373–381. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Terry R. D. Immunocytochemical studies of neurofibrillary tangles. Am J Pathol. 1981 Jul;104(1):77–89. [PMC free article] [PubMed] [Google Scholar]