Abstract
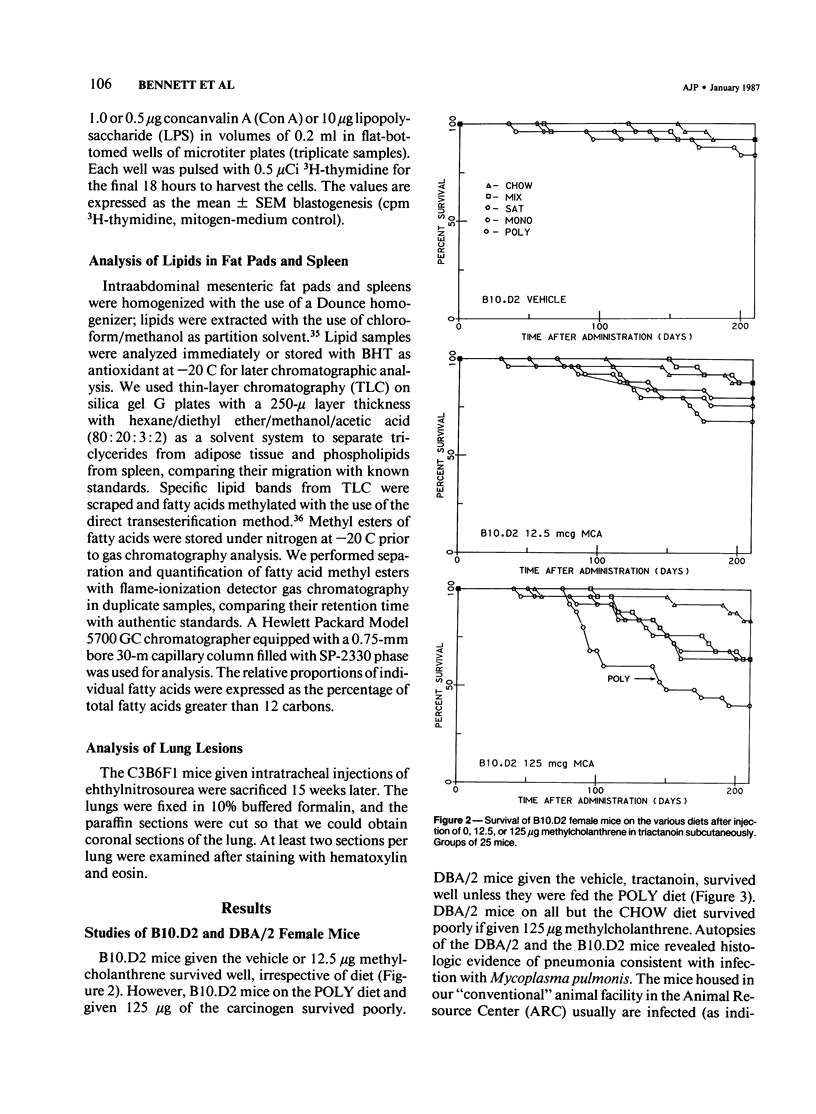
To test whether or not diets enriched in w-6 polyunsaturated fatty acids are significantly immunosuppressive, B10.D2, DBA/2, and C3B6F1 mice were fed diets enriched for fatty acids: linoleic (POLY), oleic (MONO), palmitic (SAT), or eicosapentanoic (FISH). The B10.D2 and DBA/2 mice were given injected methylcholanthrene several weeks later, and immune studies were performed several months after carcinogen treatment. In conventional quarters, DBA/2 fed the POLY diet survived poorly, and many were infected with Mycoplasma pulmonis, even if given the vehicle, tractinoin, only. B10.D2 mice survived well unless on the POLY diet and given methylcholanthrene. Nevertheless, only mice on the POLY diet were significantly immunosuppressed, and only T-cell-mediated cutaneous sensitivity reactions were affected. Antibody, natural killer cell, and natural cytotoxic cell responses were not influenced by the diets. The C3B6F1 mice were assessed for immune functions prior to carcinogen (ethylnitrosourea) instillation into the trachea, and no immunosuppression was detected. After instillation, mice on the POLY and MONO diets were suppressed for T-cell cutaneous responses. Deliberate infection with Mycoplasma pulmonis resulted in suppressed cutaneous T-cell responses in the POLY group of C3B6F1 mice, and aspirin partially reversed the immunosuppression. Mice on the FISH diet were resistant to immunosuppression. It is tentatively concluded that diets rich in w-6 polyunsaturated diets, while not directly immunosuppressive, do predispose animals to suppression of certain T-cell-mediated immune responses. This immunosuppression can be "triggered" by infection and/or by exposure to carcinogens.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal B. R., Rhoads J. E., Jr, Bansal S. C. Effects of diet on colon carcinogenesis and the immune system in rats treated with 1,2-dimethylhydrazine. Cancer Res. 1978 Oct;38(10):3293–3303. [PubMed] [Google Scholar]
- Broitman S. A., Vitale J. J., Vavrousek-Jakuba E., Gottlieb L. S. Polyunsaturated fat, cholesterol and large bowel tumorigenesis. Cancer. 1977 Nov;40(5 Suppl):2455–2463. doi: 10.1002/1097-0142(197711)40:5+<2455::aid-cncr2820400911>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Carroll K. K., Khor H. T. Dietary fat in relation to tumorigenesis. Prog Biochem Pharmacol. 1975;10:308–353. [PubMed] [Google Scholar]
- Carroll K. K., Khor H. T. Effects of level and type of dietary fat on incidence of mammary tumors induced in female Sprague-Dawley rats by 7,12-dimethylbenz()anthracene. Lipids. 1971 Jun;6(6):415–420. doi: 10.1007/BF02531379. [DOI] [PubMed] [Google Scholar]
- Carter C. A., Milholland R. J., Shea W., Ip M. M. Effect of the prostaglandin synthetase inhibitor indomethacin on 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats fed different levels of fat. Cancer Res. 1983 Aug;43(8):3559–3562. [PubMed] [Google Scholar]
- Cartwright I. J., Pockley A. G., Galloway J. H., Greaves M., Preston F. E. The effects of dietary omega-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis. 1985 Jun;55(3):267–281. doi: 10.1016/0021-9150(85)90106-6. [DOI] [PubMed] [Google Scholar]
- Clifford C. K., Smith L. M., Erickson K. L., Hamblin C. L., Creveling R. K., Clifford A. J. Effect of dietary triglycerides on lymphocyte transformation in rats. J Nutr. 1983 Mar;113(3):669–679. doi: 10.1093/jn/113.3.669. [DOI] [PubMed] [Google Scholar]
- Culp B. R., Titus B. G., Lands W. E. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979 Nov;3(5):269–278. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- DeWille J. W., Fraker P. J., Romsos D. R. Effects of essential fatty acid deficiency, and various levels of dietary polyunsaturated fatty acids, on humoral immunity in mice. J Nutr. 1979 Jun;109(6):1018–1027. doi: 10.1093/jn/109.6.1018. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Erickson K. L., McNeill C. J., Gershwin M. E., Ossmann J. B. Influence of dietary fat concentration and saturation on immune ontogeny in mice. J Nutr. 1980 Aug;110(8):1555–1572. doi: 10.1093/jn/110.8.1555. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Gross R. L., Newberne P. M. Role of nutrition in immunologic function. Physiol Rev. 1980 Jan;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr The effects of unsaturated dietary fats on absorption, excretion, synthesis, and distribution of cholesterol in man. J Clin Invest. 1970 Jun;49(6):1135–1152. doi: 10.1172/JCI106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard L. A., Abraham S. Effect of dietary polyunsaturated fatty acids on growth of mammary adenocarcinomas in mice and rats. Cancer Res. 1979 Nov;39(11):4430–4437. [PubMed] [Google Scholar]
- Hurd E. R., Johnston J. M., Okita J. R., MacDonald P. C., Ziff M., Gilliam J. W. Prevention of glomerulonephritis and prolonged survival in New Zealand Black/New Zealand White F1 hybrid mice fed an essential fatty acid-deficient diet. J Clin Invest. 1981 Feb;67(2):476–485. doi: 10.1172/JCI110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C., Carter C. A., Ip M. M. Requirement of essential fatty acid for mammary tumorigenesis in the rat. Cancer Res. 1985 May;45(5):1997–2001. [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kollmorgen G. M., Sansing W. A., Lehman A. A., Fischer G., Longley R. E., Alexander S. S., Jr, King M. M., McCay P. B. Inhibition of lymphocyte function in rats fed higher-fat diets. Cancer Res. 1979 Sep;39(9):3458–3462. [PubMed] [Google Scholar]
- Kouri R. E., Ratrie H., Whitmire C. E. Evidence of a genetic relationship between susceptibility to 3-methyl-cholanthrene-induced subcutaneous tumors and inducibility of aryl hydrocarbon hydroxylase. J Natl Cancer Inst. 1973 Jul;51(1):197–200. doi: 10.1093/jnci/51.1.197. [DOI] [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J., Bennett M., Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979 Oct;123(4):1832–1838. [PubMed] [Google Scholar]
- Landymore R. W., Kinley C. E., Cooper J. H., MacAulay M., Sheridan B., Cameron C. Cod-liver oil in the prevention of intimal hyperplasia in autogenous vein grafts used for arterial bypass. J Thorac Cardiovasc Surg. 1985 Mar;89(3):351–357. [PubMed] [Google Scholar]
- Lattime E. C., Pecoraro G. A., Stutman O. The activity of natural cytotoxic cells is augmented by interleukin 2 and interleukin 3. J Exp Med. 1983 Mar 1;157(3):1070–1075. doi: 10.1084/jem.157.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage G., Roy C. C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986 Jan;27(1):114–120. [PubMed] [Google Scholar]
- Levy J. A., Ibrahim A. B., Shirai T., Ohta K., Nagasawa R., Yoshida H., Estes J., Gardner M. Dietary fat affects immune response, production of antiviral factors, and immune complex disease in NZB/NZW mice. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1974–1978. doi: 10.1073/pnas.79.6.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locniskar M., Nauss K. M., Newberne P. M. The effect of quality and quantity of dietary fat on the immune system. J Nutr. 1983 May;113(5):951–961. doi: 10.1093/jn/113.5.951. [DOI] [PubMed] [Google Scholar]
- Lust J. A., Kumar V., Burton R. C., Bartlett S. P., Bennett M. Heterogeneity of natural killer cells in the mouse. J Exp Med. 1981 Aug 1;154(2):306–317. doi: 10.1084/jem.154.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson F. H., Grundy S. M. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985 Feb;26(2):194–202. [PubMed] [Google Scholar]
- Meade C. J., Mertin J. The mechanism of immunoinhibition by arachidonic and linoleic acid: effects on the lymphoid and reticulo-endothelial systems. Int Arch Allergy Appl Immunol. 1976;51(1):2–24. doi: 10.1159/000231575. [DOI] [PubMed] [Google Scholar]
- Mertin J., Hughes D., Shenton B. K., Dickinson J. P. In-vitro inhibition by unsaturated fatty acids of the PPD- and PHA-induced lymphocyte response. Klin Wochenschr. 1974 Mar 1;52(5):248–250. doi: 10.1007/BF01468599. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Locniskar M., Newberne P. M. Effect of alterations in the quality and quantity of dietary fat on 1,2-dimethylhydrazine-induced colon tumorigenesis in rats. Cancer Res. 1983 Sep;43(9):4083–4090. [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Phillipson B. E., Rothrock D. W., Connor W. E., Harris W. S., Illingworth D. R. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985 May 9;312(19):1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi C., Puccetti P., Santoni A., Herberman R. B. Rapid in vivo assay of mouse natural killer cell activity. J Natl Cancer Inst. 1979 Oct;63(4):1041–1045. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Sakaguchi M., Hiramatsu Y., Takada H., Yamamura M., Hioki K., Saito K., Yamamoto M. Effect of dietary unsaturated and saturated fats on azoxymethane-induced colon carcinogenesis in rats. Cancer Res. 1984 Apr;44(4):1472–1477. [PubMed] [Google Scholar]
- Schneider E. L., Reed J. D., Jr Life extension. N Engl J Med. 1985 May 2;312(18):1159–1168. doi: 10.1056/NEJM198505023121805. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J. Allergic contact dermatitis induced by intratracheal administration of hapten. J Immunol. 1983 Oct;131(4):1748–1753. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Weyman C., Belin J., Smith A. D., Thompson R. H. Letter: Linoleic acid as an immunosuppressive agent. Lancet. 1975 Jul 5;2(7923):33–33. doi: 10.1016/s0140-6736(75)92977-3. [DOI] [PubMed] [Google Scholar]
- Wynder E. L. Nutrition and cancer. Fed Proc. 1976 May 1;35(6):1309–1315. [PubMed] [Google Scholar]
- Yu B. P., Masoro E. J., Murata I., Bertrand H. A., Lynd F. T. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982 Mar;37(2):130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]



