Abstract
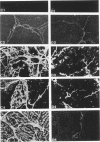
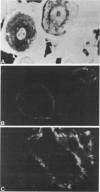
Serum assays of procollagen peptides have been suggested as useful clinical indicators of hepatic fibrogenesis. To evaluate these assays in an experimental situation, the carbon tetrachloride model of hepatic fibrosis was produced in the rat. With affinity-purified antibodies, ELISA assays were developed and used for detecting nanogram quantities of the aminopropeptides of Types I and III procollagen (pro-I and pro-III) in rat serum. Serum SGPT levels were also determined. Routine histologic staining, as well as immunofluorescent staining for localization of Types I, III, and IV collagen and the aminopropeptides of Types I and III procollagen were performed on corresponding livers. It was found that serum pro-III levels were elevated after 45 and 70 days of treatment (28 +/- 15.2 ng/ml and 21 +/- 3.4 ng/ml, respectively) but returned to normal levels after 90 days of treatment (less than 3 ng/ml). Serum pro-I levels were elevated after 45 days of treatment (1028 +/- 504 ng/ml) but were normal at 70 and 90 days of treatment. Serum SGPT values were elevated at 45 and 70 days of treatment but were normal at 90 days of treatment. The decline in serum pro-III levels appeared to parallel the noted decline in SGPT values. Linear regression analysis revealed a good correlation between pro-III levels and histologic inflammation (r = 0.886) but a poor correlation between pro-III levels and histologic fibrosis (r = 0.116). Immunohistochemistry revealed that the pro-III antigen persists extracellularly for at least 45 days. Therefore, serum assays of pro-III might reflect extracellular collagen degradation as well as active collagen secretion. This would limit the clinical utility of the pro-III assay as an unequivocal marker of active hepatic collagen deposition.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clement B., Grimaud J. A., Campion J. P., Deugnier Y., Guillouzo A. Cell types involved in collagen and fibronectin production in normal and fibrotic human liver. Hepatology. 1986 Mar-Apr;6(2):225–234. doi: 10.1002/hep.1840060212. [DOI] [PubMed] [Google Scholar]
- Colombo M., Annoni G., Donato M. F., Conte D., Martines D., Zaramella M. G., Bianchi P. A., Piperno A., Tiribelli C. Serum type III procollagen peptide in alcoholic liver disease and idiopathic hemochromatosis: its relationship to hepatic fibrosis, activity of the disease and iron overload. Hepatology. 1985 May-Jun;5(3):475–479. doi: 10.1002/hep.1840050322. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Olsen B. R., Timpl R., Perlish J. S., Lovelace O. Collagen fibril formation during embryogenesis. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei A., Zimmermann A., Weigand K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology. 1984 Sep-Oct;4(5):830–834. doi: 10.1002/hep.1840040505. [DOI] [PubMed] [Google Scholar]
- Galambos M. R., Collins D. C., Galambos J. T. A radioimmunoassay procedure for type III procollagen: its use in the detection of hepatic fibrosis. Hepatology. 1985 Jan-Feb;5(1):38–42. doi: 10.1002/hep.1840050109. [DOI] [PubMed] [Google Scholar]
- Grimaud J. A., Druguet M., Peyrol S., Chevalier O., Herbage D., El Badrawy N. Collagen immunotyping in human liver: light and electron microscope study. J Histochem Cytochem. 1980 Nov;28(11):1145–1156. doi: 10.1177/28.11.7000887. [DOI] [PubMed] [Google Scholar]
- Hahn E. G. Blood analysis for liver cirrhosis. J Hepatol. 1985;1(1):67–73. doi: 10.1016/s0168-8278(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Barwick K. W. Use of avidin-biotin complex in an ELISA system: a quantitative comparison with two other immunoperoxidase detection systems using keratin antisera. Lab Invest. 1983 Jan;48(1):98–107. [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä O., Risteli L., Sotaniemi E. A., Risteli J. Aminoterminal propeptide of type III procollagen in serum in alcoholic liver disease. Gastroenterology. 1983 Aug;85(2):254–259. [PubMed] [Google Scholar]
- Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983 Jan-Feb;3(1):112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- Rohde H., Vargas L., Hahn E., Kalbfleisch H., Bruguera M., Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979 Dec;9(6):451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Leo M. A., Lieber C. S. Ultrastructural localization of type III procollagen in baboon liver. Am J Pathol. 1986 Feb;122(2):212–217. [PMC free article] [PubMed] [Google Scholar]
- Savolainen E. R., Goldberg B., Leo M. A., Velez M., Lieber C. S. Diagnostic value of serum procollagen peptide measurements in alcoholic liver disease. Alcohol Clin Exp Res. 1984 Jul-Aug;8(4):384–389. doi: 10.1111/j.1530-0277.1984.tb05684.x. [DOI] [PubMed] [Google Scholar]
- Seyer J. M. Interstitial collagen polymorphism in rat liver with CCl4-induced cirrhosis. Biochim Biophys Acta. 1980 May 22;629(3):490–498. doi: 10.1016/0304-4165(80)90154-3. [DOI] [PubMed] [Google Scholar]
- Smith B. D., McKenney K. H., Lustberg T. J. Characterization of collagen precursors found in rat skin and rat bone. Biochemistry. 1977 Jun 28;16(13):2980–2985. doi: 10.1021/bi00632a027. [DOI] [PubMed] [Google Scholar]
- Torres-Salinas M., Parés A., Caballería J., Jiménez W., Heredia D., Bruguera M., Rodés J. Serum procollagen type III peptide as a marker of hepatic fibrogenesis in alcoholic hepatitis. Gastroenterology. 1986 May;90(5 Pt 1):1241–1246. doi: 10.1016/0016-5085(86)90391-4. [DOI] [PubMed] [Google Scholar]