Abstract
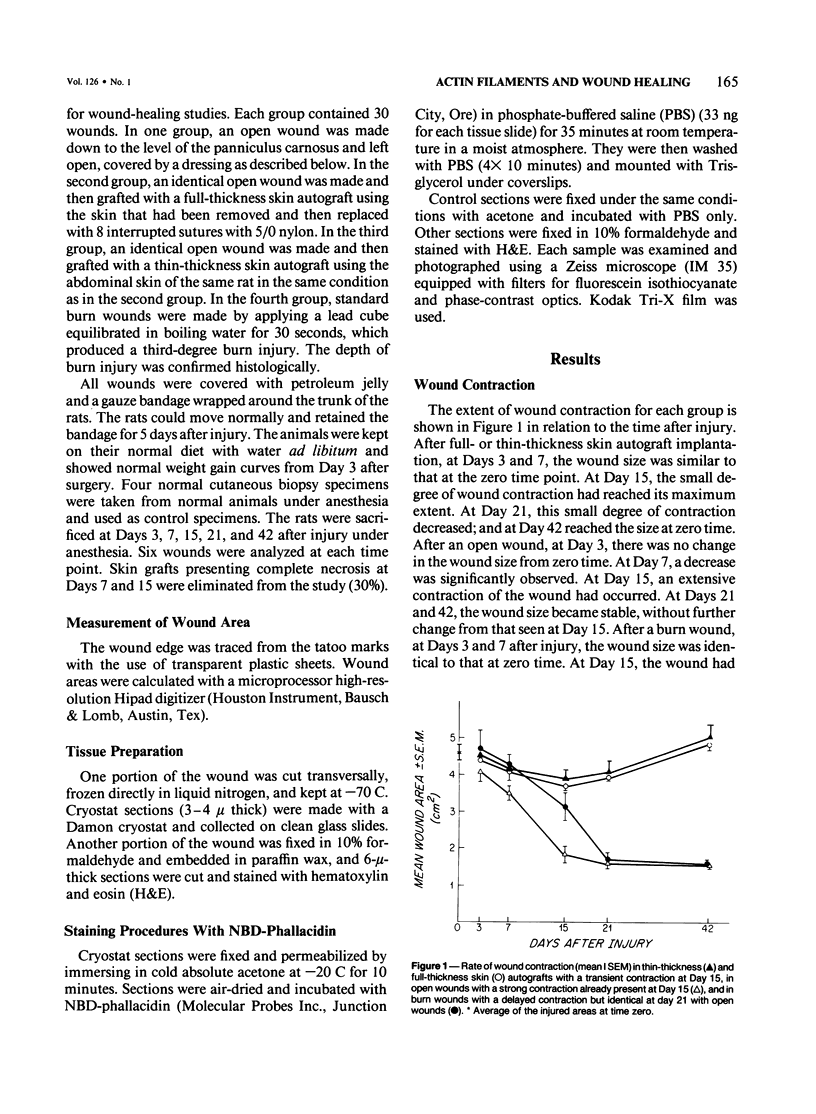
During wound healing, it has been suggested, modified fibroblasts rich in actin filaments are responsible for wound contraction. With the use of specific fluorescent probe (NBD-phallacidin), the distribution of actin filaments are compared in normal dermis and in several wound contraction models, including open and burn wounds and full and thin-thickness skin autografts. Fibroblasts of normal dermis are slightly stained with NBD-phallacidin. Fibroblasts with actin filaments are increased in autografts, particularly at Days 15 and 21 after grafting, and are prominent in open and burn wounds. The wound contraction rate is not directly related to the presence of actin-staining fibroblasts. After stabilization of the contraction of open or burn wounds, fibroblasts rich in actin filaments remain. The superficial layer of full-thickness skin graft contains a similar actin distribution without concomitant contraction. It is concluded that the distribution of actin-rich fibroblasts corresponds morphologically to previous areas of necrosis or injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., MEDAWAR P. B. Contracture and intussusceptive growth in the healing of extensive wounds in mammalian skin. J Anat. 1955 Jan;89(1):114–123. [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Are stress fibres contractile? Nature. 1981 Dec 24;294(5843):691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Grislis G., Hunt T. K. Evidence for the involvement of microtubules in wound contraction. Am J Surg. 1977 Jun;133(6):706–709. doi: 10.1016/0002-9610(77)90159-3. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Hüttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978 Mar;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J. R., Miller E. R. Histology of healing split-thickness, full-thickness autogenous skin grafts and donor sites. Arch Surg. 1965 Oct;91(4):658–670. doi: 10.1001/archsurg.1965.01320160112027. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Gabbiani G., Ryan G. B., Majno G. Fibroblasts of granulation tissue: immunofluorescent staining with antismooth muscle serum. Proc Soc Exp Biol Med. 1971 Nov;138(2):466–469. doi: 10.3181/00379727-138-35920. [DOI] [PubMed] [Google Scholar]
- Klein L., Rudolph R. 3 H-collagen turnover in skin grafts. Surg Gynecol Obstet. 1972 Jul;135(1):49–57. [PubMed] [Google Scholar]
- McGrath M. H., Hundahl S. A. The spatial and temporal quantification of myofibroblasts. Plast Reconstr Surg. 1982 Jun;69(6):975–985. doi: 10.1097/00006534-198206000-00012. [DOI] [PubMed] [Google Scholar]
- Rudolph R. Inhibition of myofibroblasts by skin grafts. Plast Reconstr Surg. 1979 Apr;63(4):473–480. doi: 10.1097/00006534-197904000-00005. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Klein L. Healing processes in skin grafts. Surg Gynecol Obstet. 1973 Apr;136(4):641–654. [PubMed] [Google Scholar]
- Rudolph R. Location of the force of wound contraction. Surg Gynecol Obstet. 1979 Apr;148(4):547–551. [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irlé C., Montandon D., Statkov P. R., Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974 Jan;5(1):55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Fluorescently labelled molecules as probes of the structure and function of living cells. Nature. 1980 Apr 3;284(5755):405–410. doi: 10.1038/284405a0. [DOI] [PubMed] [Google Scholar]




