Abstract
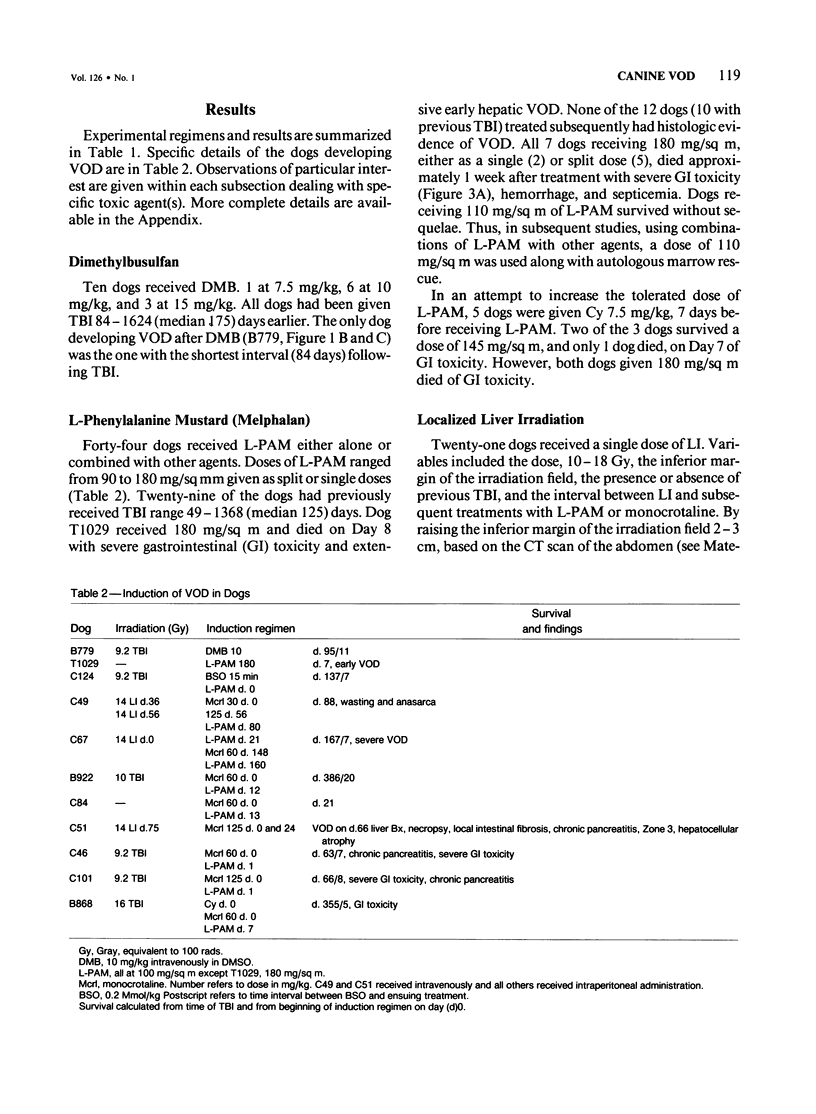
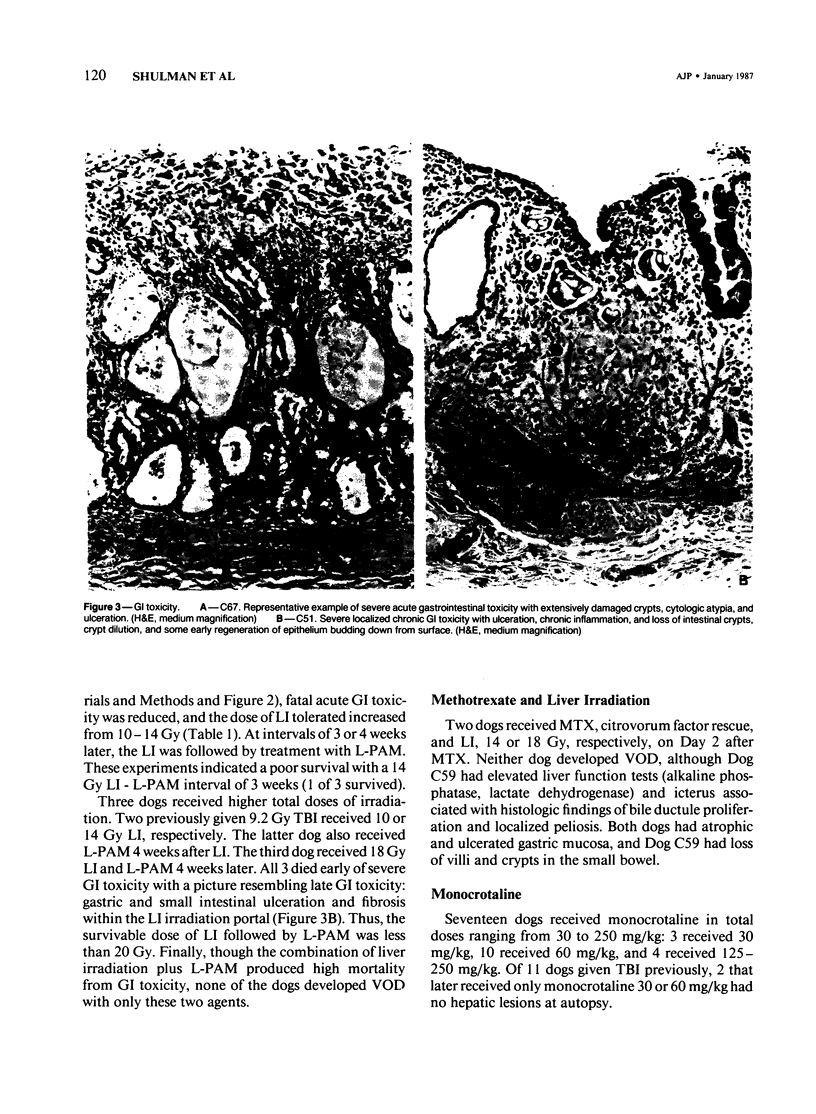
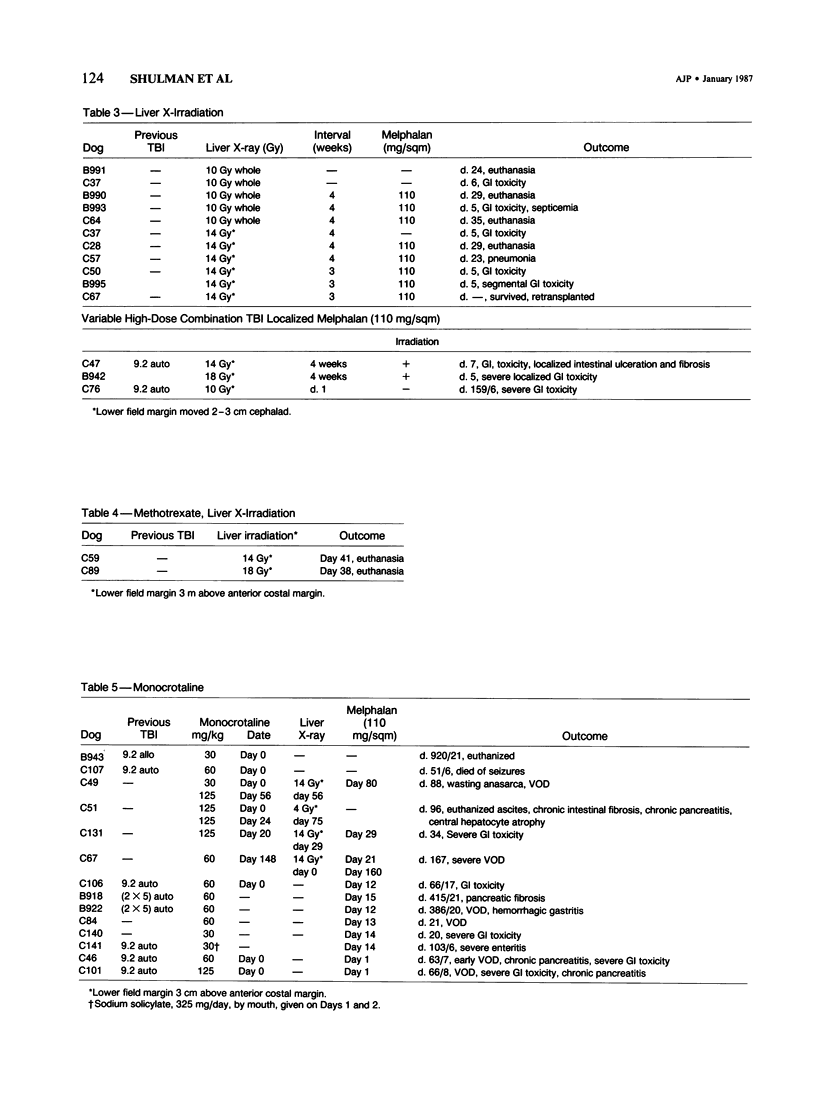
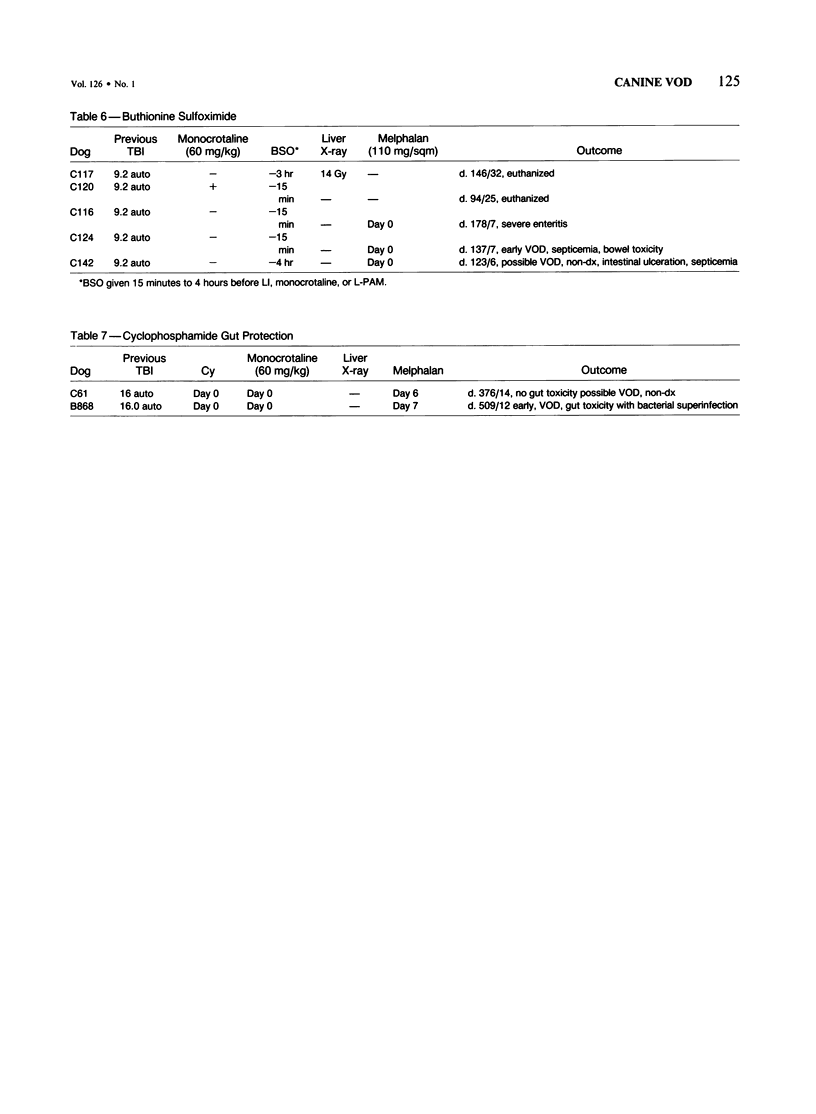
The authors attempted to induce hepatic veno-occlusive disease (VOD) in 64 dogs. Preparative treatments included combinations of total-body irradiation (TBI) or localized hepatic irradiation (LI) or both and chemotherapy consisting of dimethylbusulfan (DMB), L-phenylalanine mustard (L-PAM), methotrexate, or monocrotaline. VOD occurred infrequently in those dogs given 9.2 Gy TBI and DMB (1/10), TBI and/or LI (9.2-27 Gy) with L-PAM (2/36) or high dose methotrexate and LI (0/2). Specifically, VOD occurred in the dogs with a shorter interval between TBI and DMB or in the dog that received the glutathione reductase inhibitor, buthionine sulfoximide (BSO) before L-PAM. In contrast, among 17 dogs given monocrotaline, 8 developed VOD, particularly when used with L-PAM +/- irradiation (7/13). The major cause of death, early gastrointestinal toxicity, was further augmented by higher doses of irradiation, by shortening the interval between LI and L-PAM administration to less than 4 weeks, and administering BSO or monocrotaline before L-PAM. Gastrointestinal toxicity was lessened by giving low dose cyclophosphamide given before L-PAM. VOD can be produced in dogs especially with monocrotaline or BSO given before and L-PAM +/- irradiation. However, gastrointestinal toxicity renders the study of VOD beyond the acute phase difficult. Nevertheless, this approach appears useful for the study of VOD in other animals and for developing agents aimed at preventing VOD.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Carstens L. A., Katagiri G. J. Hepatic veins of monkeys with veno-occlusive disease. Sequential ultrastructural changes. Arch Pathol. 1969 Mar;87(3):279–289. [PubMed] [Google Scholar]
- Beschorner W. E., Pino J., Boitnott J. K., Tutschka P. J., Santos G. W. Pathology of the liver with bone marrow transplantation. Effects of busulfan, carmustine, acute graft-versus-host disease, and cytomegalovirus infection. Am J Pathol. 1980 May;99(2):369–386. [PMC free article] [PubMed] [Google Scholar]
- Bleyer W. A. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978 Jan;41(1):36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bradley E. W., Alderson P. O., Deye J. A., Mendenhall K. G., Fisher M. P., Vieras F., Rogers C. C. Effects of fractionated doses of fast neutrons and photons on the normal canine lung: relative biological effectiveness values obtained by radionuclide studies. Int J Radiat Oncol Biol Phys. 1979 Feb;5(2):197–207. doi: 10.1016/0360-3016(79)90720-x. [DOI] [PubMed] [Google Scholar]
- Deeg H. J., Storb R., Weiden P. L., Schumacher D., Shulman H., Graham T., Thomas E. D. High-dose total-body irradiation and autologous marrow reconstitution in dogs: dose-rate-related acute toxicity and fractionation-dependent long-term survival. Radiat Res. 1981 Nov;88(2):385–391. [PubMed] [Google Scholar]
- Fajardo L. F., Colby T. V. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med. 1980 Nov;104(11):584–588. [PubMed] [Google Scholar]
- Geraci J. P., Jackson K. L., Mariano M. S., Leitch J. M. Hepatic injury after whole-liver irradiation in the rat. Radiat Res. 1985 Mar;101(3):508–518. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. J., Storb R., Weiden P. L., Ochs H. D., Kolb H., Graham T. C., Floersheim G. L., Thomas E. D. Immunologic, toxicologic and marrow transplantation studies in dogs given dimethyl myleran. Biomedicine. 1974 Sep;20(5):341–351. [PubMed] [Google Scholar]
- McDonald G. B., Sharma P., Matthews D. E., Shulman H. M., Thomas E. D. The clinical course of 53 patients with venocclusive disease of the liver after marrow transplantation. Transplantation. 1985 Jun;39(6):603–608. doi: 10.1097/00007890-198506000-00005. [DOI] [PubMed] [Google Scholar]
- McDonald G. B., Sharma P., Matthews D. E., Shulman H. M., Thomas E. D. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984 Jan-Feb;4(1):116–122. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- McDonald G. B., Shulman H. M., Sullivan K. M., Spencer G. D. Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology. 1986 Feb;90(2):460–477. doi: 10.1016/0016-5085(86)90949-2. [DOI] [PubMed] [Google Scholar]
- McDonald G. B., Shulman H. M., Sullivan K. M., Spencer G. D. Intestinal and hepatic complications of human bone marrow transplantation. Part II. Gastroenterology. 1986 Mar;90(3):770–784. doi: 10.1016/0016-5085(86)91137-6. [DOI] [PubMed] [Google Scholar]
- McLean E. K. The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev. 1970 Dec;22(4):429–483. [PubMed] [Google Scholar]
- Millar J. L., Phelps T. A., Carter R. L., McElwain T. J. Cyclophosphamide pretreatment reduces the toxic effect of high dose melphalan on intestinal epithelium in sheep. Eur J Cancer. 1978 Nov;14(11):1283–1285. doi: 10.1016/0014-2964(78)90236-0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. P., Walker I. G. Studies on the cytotoxicity of Myleran and dimethyl Myleran. Can J Biochem. 1972 Oct;50(10):1074–1081. doi: 10.1139/o72-148. [DOI] [PubMed] [Google Scholar]
- Nesbit M., Krivit W., Heyn R., Sharp H. Acute and chronic effects of methotrexate on hepatic, pulmonary, and skeletal systems. Cancer. 1976 Feb;37(2 Suppl):1048–1057. doi: 10.1002/1097-0142(197602)37:2+<1048::aid-cncr2820370811>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Petry T. W., Bowden G. T., Huxtable R. J., Sipes I. G. Characterization of hepatic DNA damage induced in rats by the pyrrolizidine alkaloid monocrotaline. Cancer Res. 1984 Apr;44(4):1505–1509. [PubMed] [Google Scholar]
- Segall H. J., Wilson D. W., Dallas J. L., Haddon W. F. trans-4-Hydroxy-2-hexenal: a reactive metabolite from the macrocyclic pyrrolizidine alkaloid senecionine. Science. 1985 Aug 2;229(4712):472–475. doi: 10.1126/science.4012327. [DOI] [PubMed] [Google Scholar]
- Shulman H. M., McDonald G. B., Matthews D., Doney K. C., Kopecky K. J., Gauvreau J. M., Thomas E. D. An analysis of hepatic venocclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980 Dec;79(6):1178–1191. [PubMed] [Google Scholar]
- Thomas E. D., LeBlond R., Graham T., Storb R. Marrow infusions in dogs given midlethal or lethal irradiation. Radiat Res. 1970 Jan;41(1):113–124. [PubMed] [Google Scholar]
- Weiden P. L., Storb R., Shulman H., Graham T. C. Dimethyl myleran and autologous marrow grafting for the treatment of spontaneous canine lymphoma. Eur J Cancer. 1977 Dec;13(12):1411–1415. doi: 10.1016/0014-2964(77)90154-2. [DOI] [PubMed] [Google Scholar]
- Zook B. C., Bradley E. W., Casarett G. W., Hitzelberg R. A., Rogers C. C. The pathologic effects of fractionated fast neutrons or photons on canine liver. Cancer Clin Trials. 1981;4(1):47–65. [PubMed] [Google Scholar]
- Zook B. C., Bradley E. W., Casarett G. W., Rogers C. C. Pathologic effects of fractionated fast neutrons or photons on the pancreas, pylorus and duodenum of dogs. Int J Radiat Oncol Biol Phys. 1983 Oct;9(10):1493–1504. doi: 10.1016/0360-3016(83)90324-3. [DOI] [PubMed] [Google Scholar]