Abstract
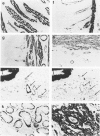
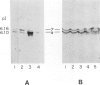
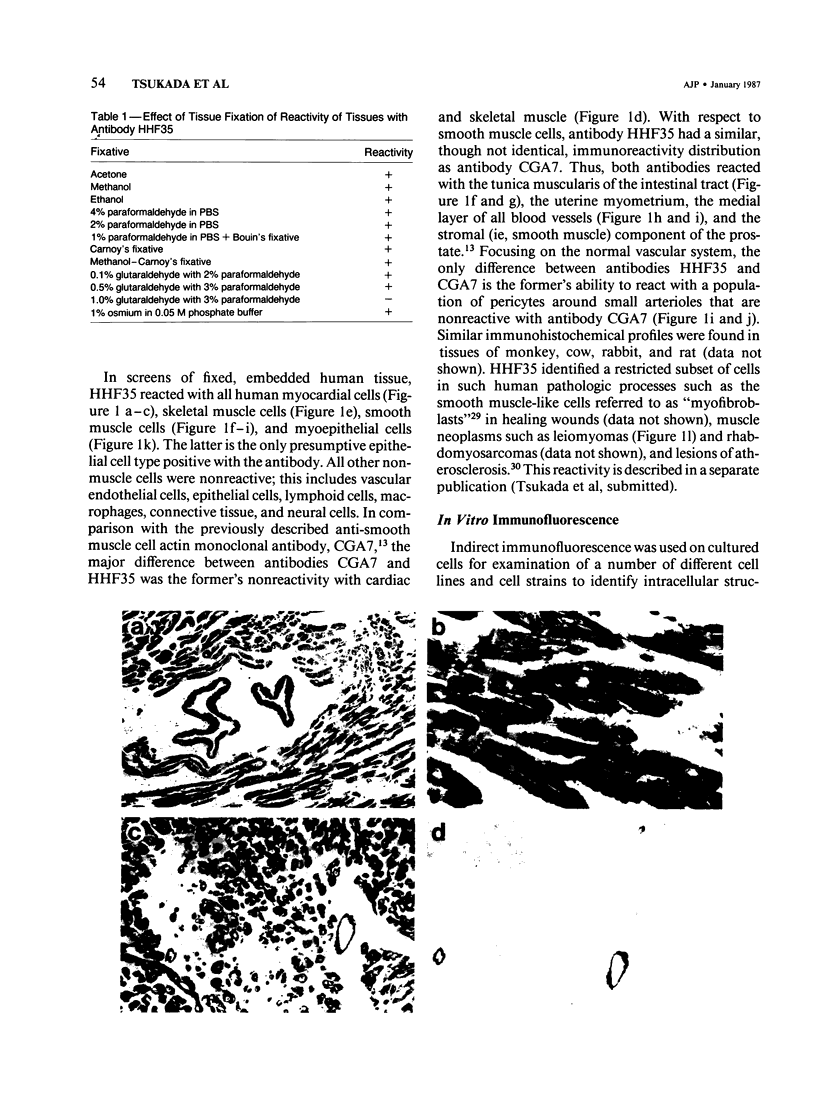
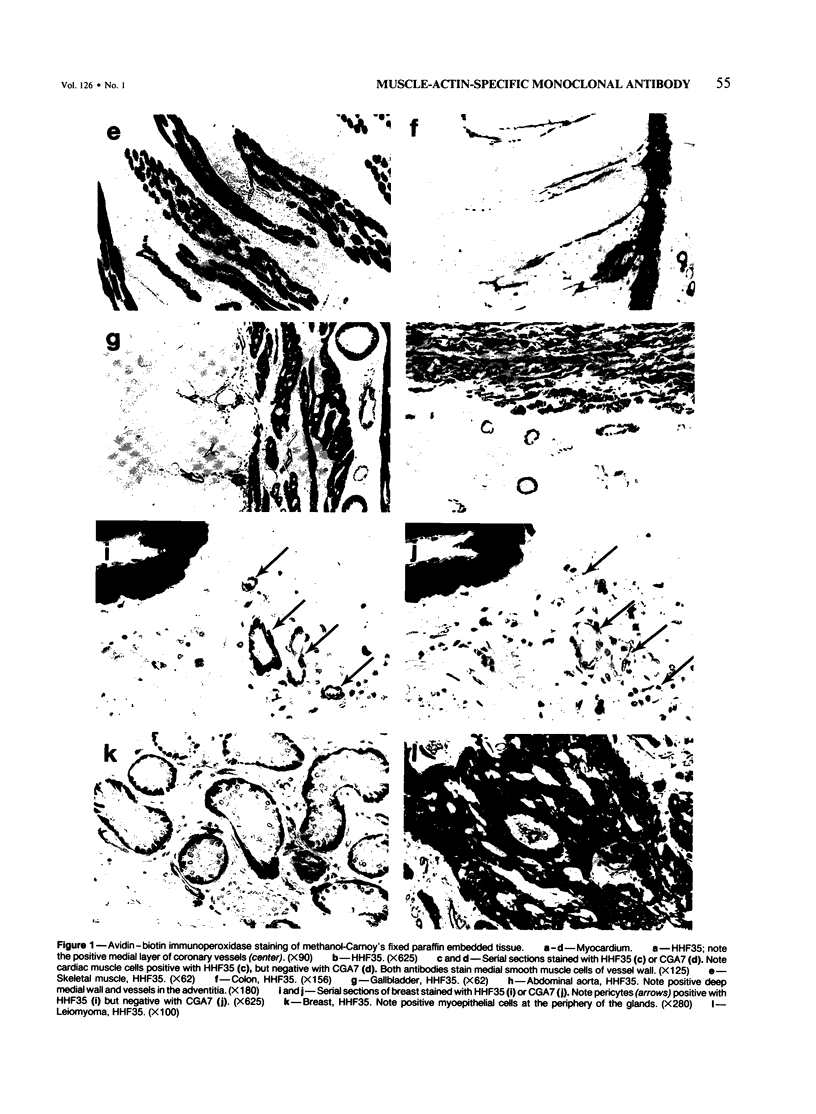
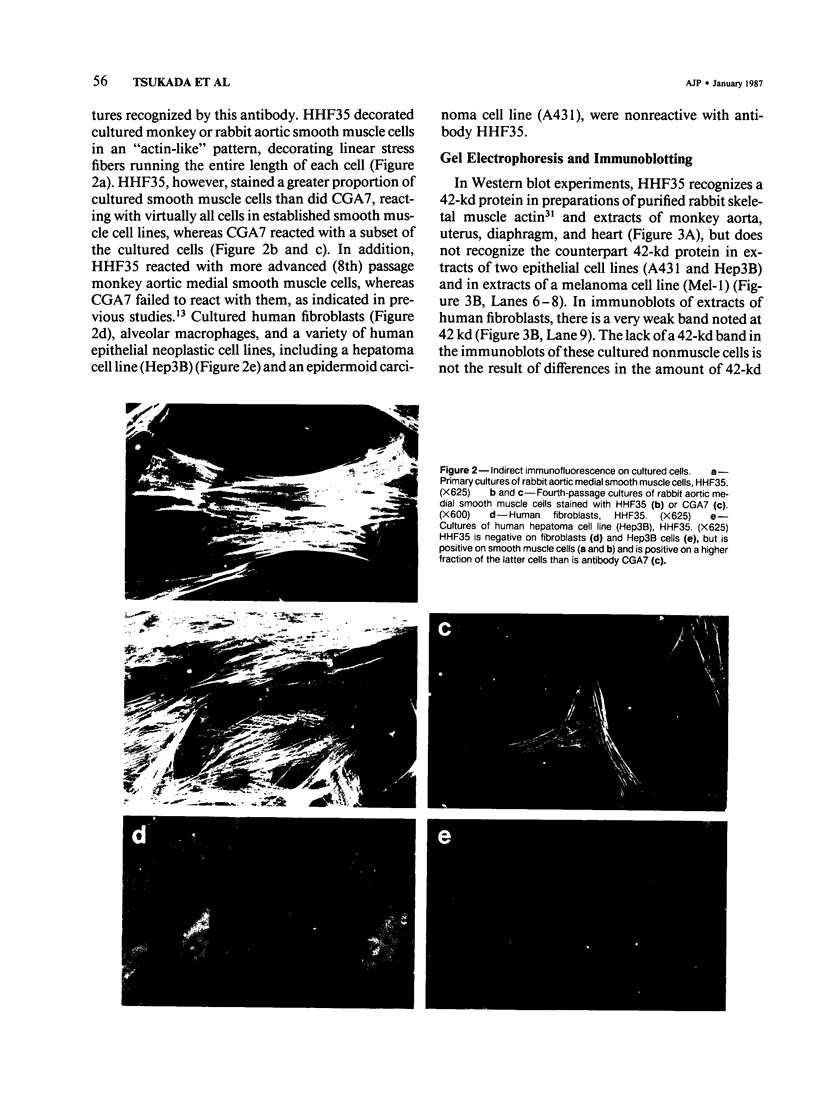
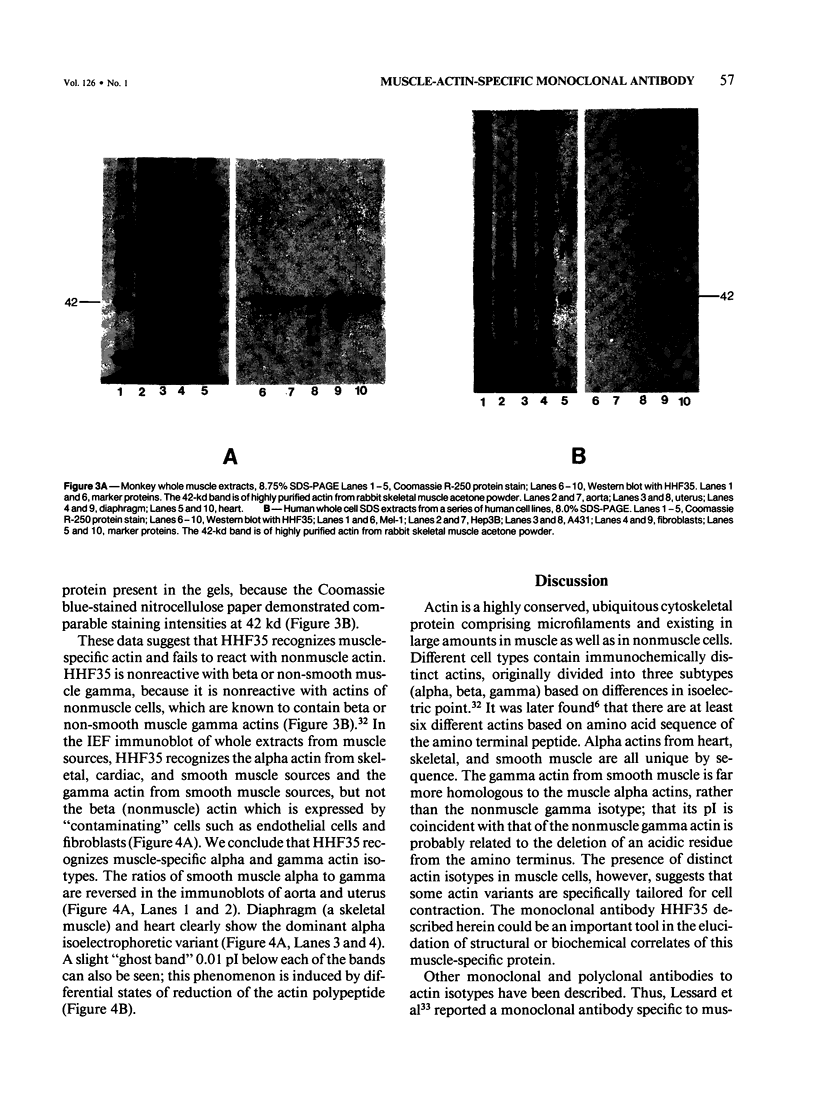
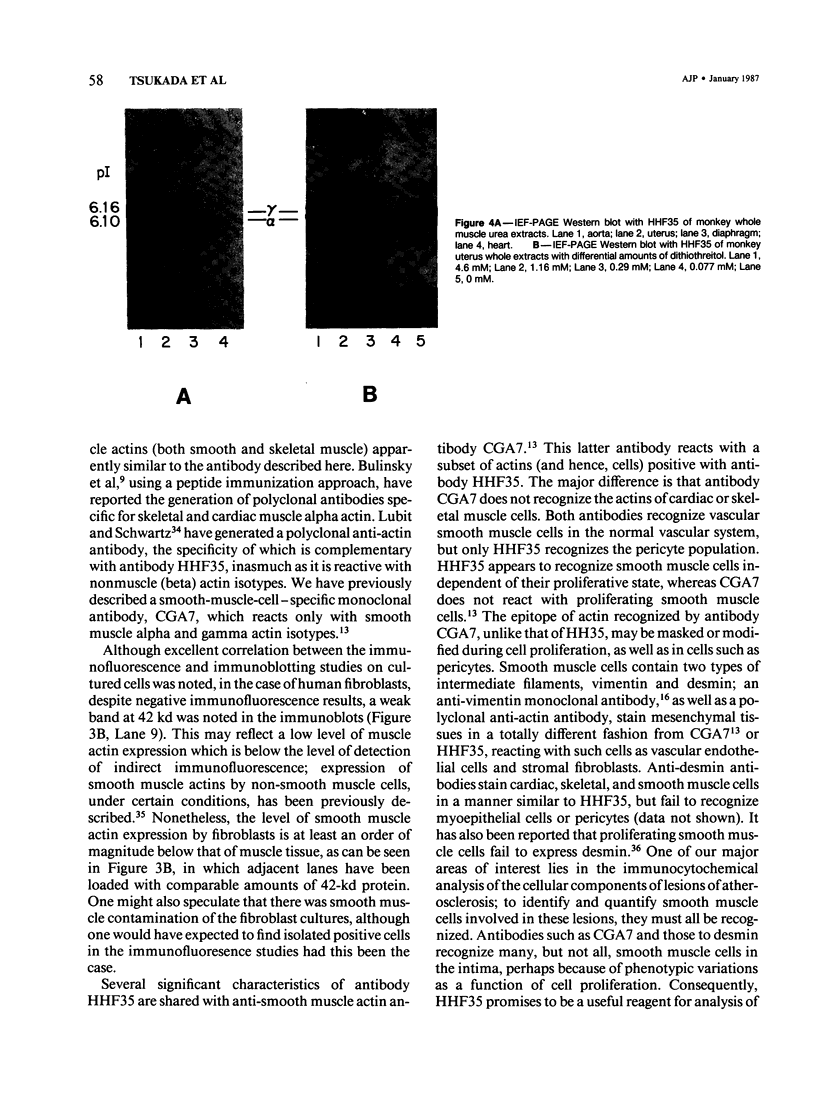
A monoclonal antibody to muscle cell actin isotypes was produced and characterized. Immunocytochemical analysis of methanol-Carnoy's-fixed, paraffin-embedded human tissue revealed that this antibody, termed HHF35, reacts with skeletal muscle cells, cardiac muscle cells, smooth muscle cells, pericytes, and myoepithelial cells, but is nonreactive with endothelial, epithelial, neural, or connective tissue cells. When assayed by indirect immunofluorescence, HHF35 reacts with microfilament bundles from various cultured mammalian smooth muscle cells, but does not react with cultured human dermal fibroblasts or various epithelial tumor cell lines. In one-dimensional gel electrophoresis immunoblot experiments this antibody detects a 42-kd polypeptide from tissue extracts of uterus, ileum, aorta, diaphragm, and heart and extract from smooth muscle cells. The antibody also reacts with a comigrating 42-kd band of highly purified rabbit skeletal muscle actin. HHF35 is nonreactive on immunoblots of extracts from all tested nonmuscle cell extracts. Immunoelectrophoresis followed by immunoblotting performed in the presence of urea and reducing agents reveals recognition of the alpha isoelectrophoretic variant of actin from skeletal, cardiac, and smooth muscle sources and of the gamma variant from smooth muscle sources. Because HHF35 reacts with virtually all muscle cells, it will be useful as a marker for muscle and muscle-derived cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun D. K., Pereira L., Norrild B., Roizman B. Application of denatured, electrophoretically separated, and immobilized lysates of herpes simplex virus-infected cells for detection of monoclonal antibodies and for studies of the properties of viral proteins. J Virol. 1983 Apr;46(1):103–112. doi: 10.1128/jvi.46.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Kumar S., Titani K., Hauschka S. D. Peptide antibody specific for the amino terminus of skeletal muscle alpha-actin. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1506–1510. doi: 10.1073/pnas.80.6.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Charbord P., Gown A. M., Keating A., Singer J. W. CGA-7 and HHF, two monoclonal antibodies that recognize muscle actin and react with adherent cells in human long-term bone marrow cultures. Blood. 1985 Nov;66(5):1138–1142. [PubMed] [Google Scholar]
- Chizzonite R. A., Everett A. W., Prior G., Zak R. Comparison of myosin heavy chains in atria and ventricles from hyperthyroid, hypothyroid, and euthyroid rabbits. J Biol Chem. 1984 Dec 25;259(24):15564–15571. [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L. A chemical comparison of tropomyosins from muscle and non-muscle tissues. J Mol Biol. 1975 Jul 5;95(3):447–454. doi: 10.1016/0022-2836(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gorza L., Sartore S., Thornell L. E., Schiaffino S. Myosin types and fiber types in cardiac muscle. III. Nodal conduction tissue. J Cell Biol. 1986 May;102(5):1758–1766. doi: 10.1083/jcb.102.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Kasamatsu H. On the electrotransfer of polypeptides from gels to nitrocellulose membranes. Anal Biochem. 1983 Feb 1;128(2):302–311. doi: 10.1016/0003-2697(83)90379-2. [DOI] [PubMed] [Google Scholar]
- Lubit B. W., Schwartz J. H. Immunological characterization of an anti-actin antibody specific for cytoplasmic actins and its use for the immunocytological localization of actin in Aplysia nervous tissue. J Histochem Cytochem. 1983 Jun;31(6):728–736. doi: 10.1177/31.6.6404982. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Kalnoski M. H., Lessard J. L., Bulinski J. C. Immunolocalization of the gamma isoform of nonmuscle actin in cultured cells. J Cell Biol. 1986 May;102(5):1726–1737. doi: 10.1083/jcb.102.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M., Wieczorek D. F., Nadal-Ginard B. Characterization of a developmentally regulated perinatal myosin heavy-chain gene expressed in skeletal muscle. J Biol Chem. 1984 Nov 10;259(21):13573–13578. [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M. Myosin from fetal hearts contains the skeletal muscle embryonic light chain. Nature. 1980 Aug 14;286(5774):731–733. doi: 10.1038/286731a0. [DOI] [PubMed] [Google Scholar]