Abstract
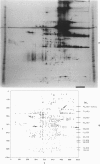
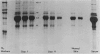
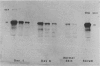
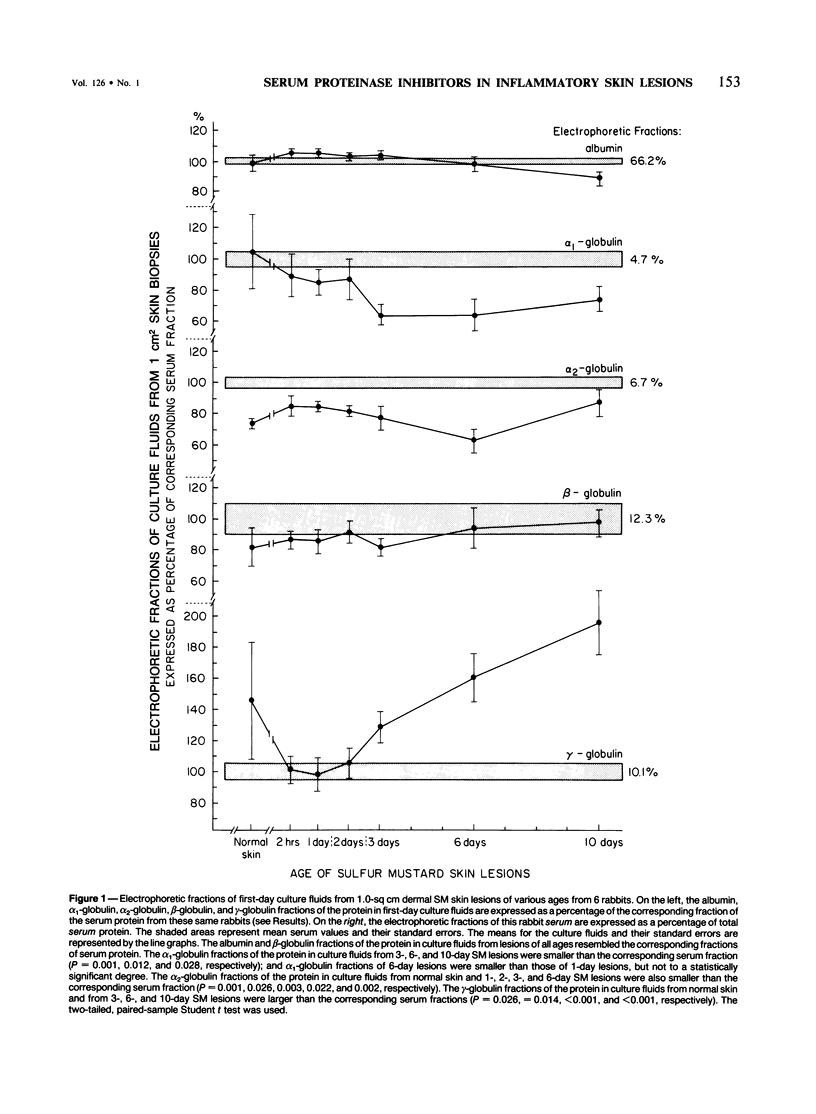
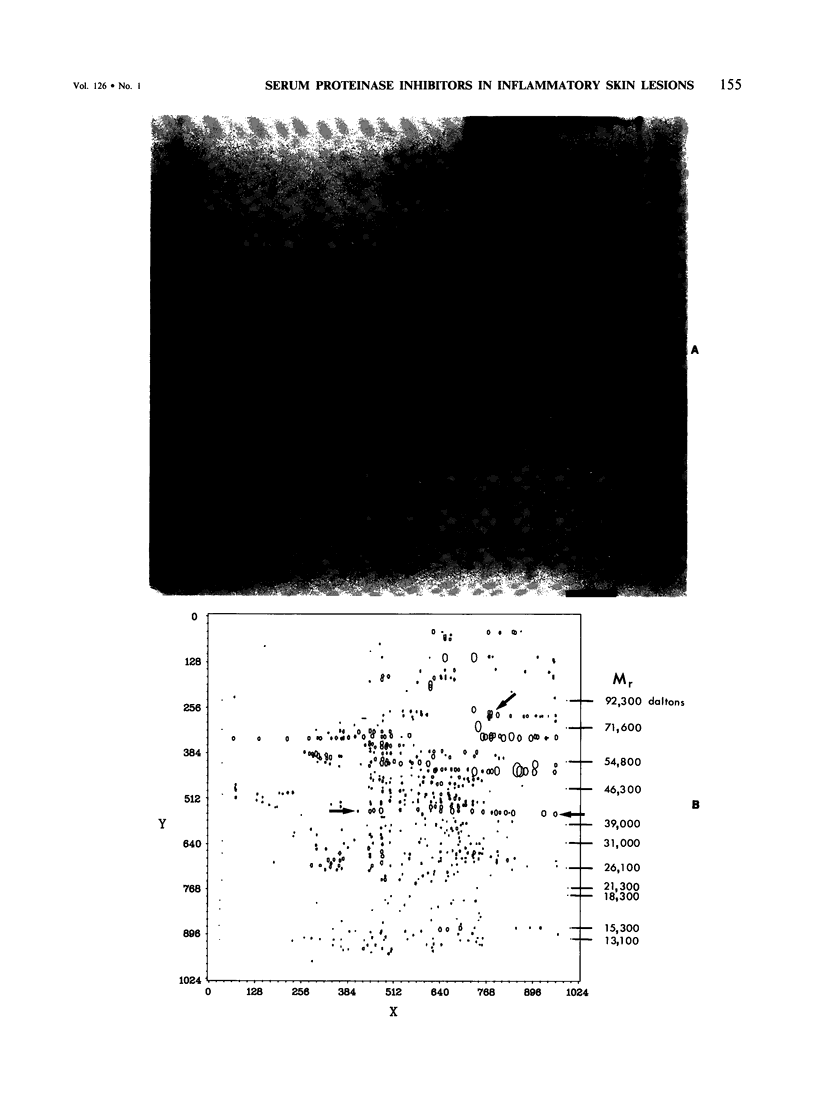
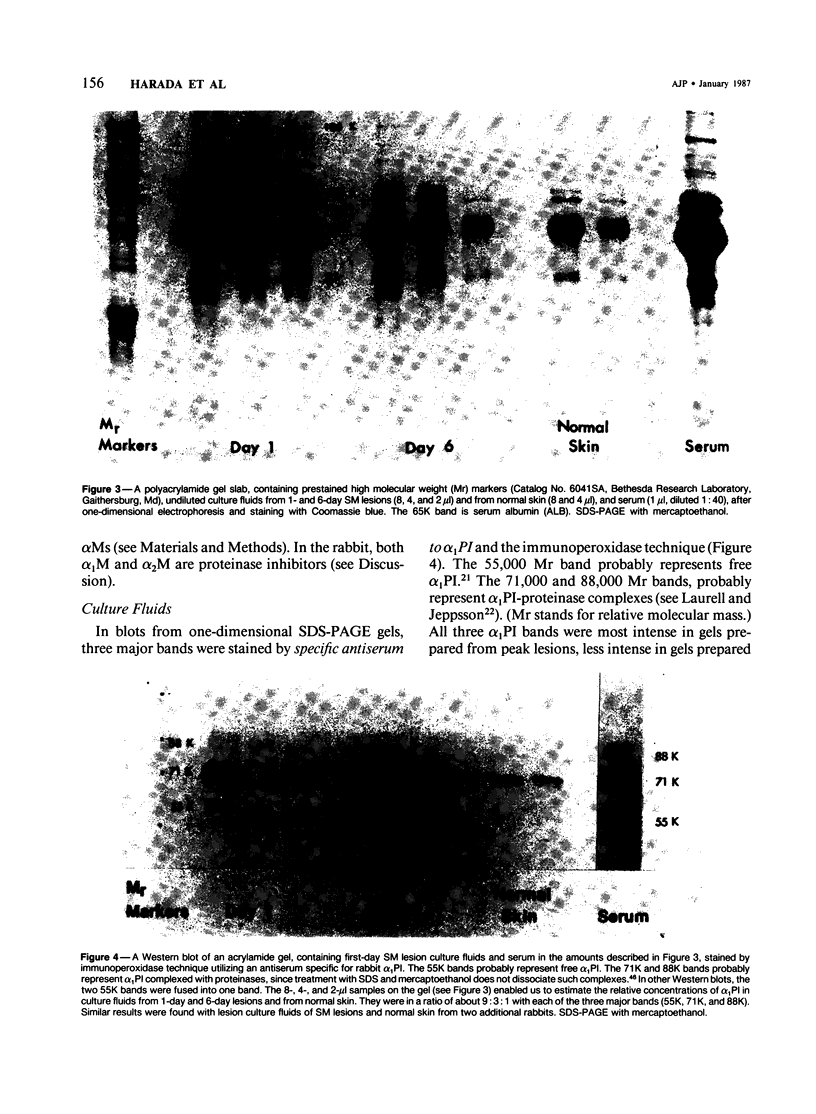
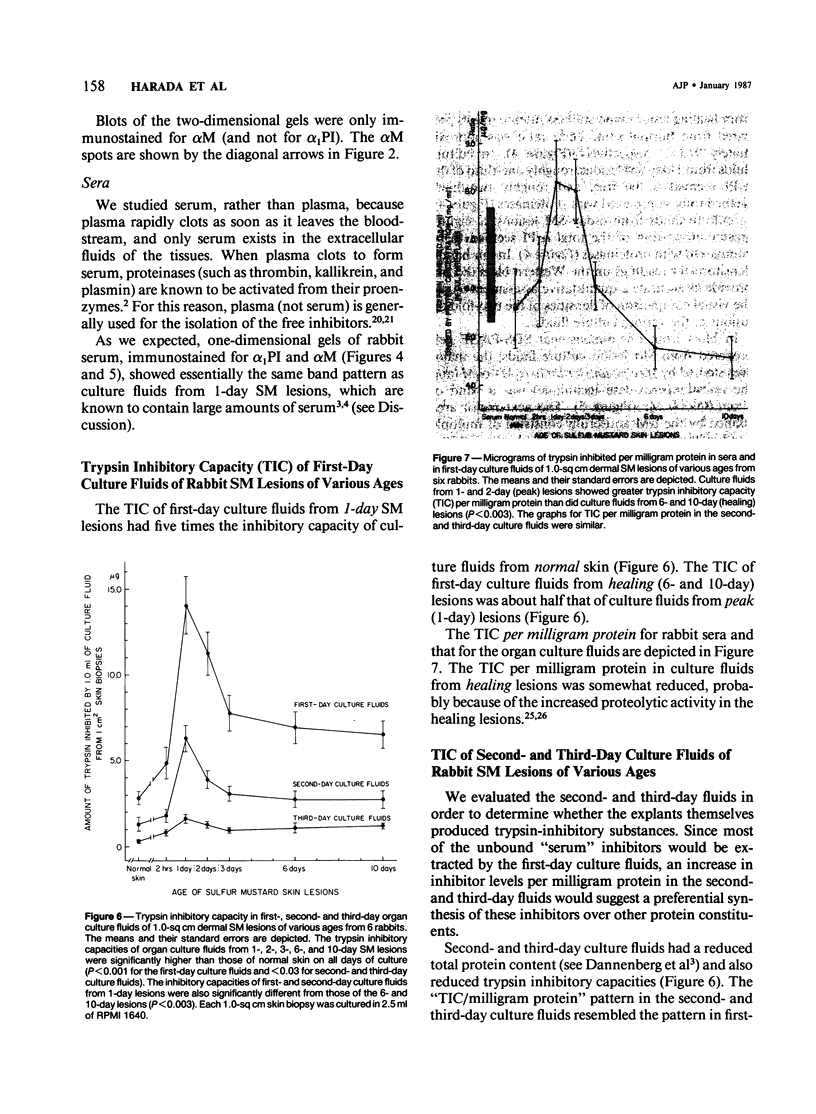
This is the third report in a series on the inflammatory mediators and modulators released in organ culture from skin lesions of various ages, which were produced in vivo in rabbits by the military vesicant, sulfur mustard (SM). It describes the electrophoretic protein fractions and trypsin-inhibitory capacities of the various culture fluids and the amounts of alpha 1-proteinase inhibitor and alpha-macroglobulin proteinase inhibitors in these fluids. With one-dimensional electrophoresis, the albumin and beta-globulin fractions of protein in culture fluids varied little with the development and healing of the SM lesions. These fractions proportionally resembled the corresponding fractions found in serum. The alpha 1-globulin fraction was proportionally smaller than the corresponding fractions of serum as the lesions healed. The alpha 2-globulin fraction was proportionally smaller than the corresponding fractions of serum at all stages of lesion development and healing. The gamma-globulin fraction was proportionally larger as the lesions healed. With two-dimensional electrophoresis, about 68%, 46%, and 35% of the protein spots in culture fluids from representative 1-day and 6-day SM lesions and normal skin, respectively, matched those from serum. In each case, the large, diffuse, serum albumin spot represented about two-thirds of the protein present. Thus, gravimetrically, in normal skin and in both developing and healing lesions, the extracellular proteins were 80-90% of serum origin. The trypsin-inhibitory capacity (TIC) per milligram protein in the culture fluids of healing lesions was markedly less than the TIC per milligram protein in the fluids of peak lesions. This decrease correlates well with the decrease found in the alpha 1-globulin fraction, which contains alpha 1-antiproteinase (alpha 1-PI) (and alpha 1-macroglobulin [alpha 1M] in rabbits). The alpha 1PI and the alpha 1M-alpha 2M proteinase inhibitors were identified in the culture fluids by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blots, specific antibodies, and the immuno-peroxidase technique. The levels of both free and proteinase-complexed alpha 1PI and alpha M inhibitors in the culture fluids decreased as the lesions healed. In both developing and healing lesions, at least half of the alpha 1PI and alpha M inhibitors seemed to be complexed with proteinases. Thus, serum seems to be a major source of unbounded extracellular protein within acute inflammatory lesions, and serum proteinase inhibitors seem to be the host's major defense against local damage by proteinases from serum, infiltrating leukocytes, and activated fibroblasts.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L., Tollaksen S. L. Proteins of human urine. I. Concentration and analysis by two-dimensional electrophoresis. Clin Chem. 1979 Jul;25(7):1199–1210. [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne B. H., Dray S., Knight K. L. Immunological relationships of serum alpha-macroglobulins in the human, rat, and rabbit. Proc Soc Exp Biol Med. 1971 Nov;138(2):531–535. doi: 10.3181/00379727-138-35933. [DOI] [PubMed] [Google Scholar]
- Boldt D. H., Chan S. K., Keaton K. Cell surface alpha 1-protease inhibitor on human peripheral mononuclear cells in culture. J Immunol. 1982 Nov;129(5):1830–1836. [PubMed] [Google Scholar]
- Böhm N. alpha 1-antitrypsin and its deficiency states. Pathol Res Pract. 1980;168(1-3):1–16. doi: 10.1016/s0344-0338(80)80204-4. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Pula P. J., Liu L. H., Harada S., Tanaka F., Vogt R. F., Jr, Kajiki A., Higuchi K. Inflammatory mediators and modulators released in organ culture from rabbit skin lesions produced in vivo by sulfur mustard. I. Quantitative histopathology; PMN, basophil, and mononuclear cell survival; and unbound (serum) protein content. Am J Pathol. 1985 Oct;121(1):15–27. [PMC free article] [PubMed] [Google Scholar]
- Davies P., Bonney R. J. Secretory products of mononuclear phagocytes: a brief review. J Reticuloendothel Soc. 1979 Jul;26(1):37–47. [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Uptake of proteinase-alpha-macroglobulin complexes by macrophages. Biochim Biophys Acta. 1975 Dec 5;411(2):295–304. doi: 10.1016/0304-4165(75)90309-8. [DOI] [PubMed] [Google Scholar]
- Dolovich J., Debanne M. T., Bell R. The role of alpha1-antitrypsin and alpha-macroglobulins in the uptake of proteinase by rabbit alveolar macrophages. Am Rev Respir Dis. 1975 Oct;112(4):521–525. doi: 10.1164/arrd.1975.112.4.521. [DOI] [PubMed] [Google Scholar]
- Feinman R. D., Wang D., Windwer S. R., Wu K. The role of enzyme lysyl amino groups in the reaction with alpha 2-macroglobulin. Ann N Y Acad Sci. 1983;421:178–187. doi: 10.1111/j.1749-6632.1983.tb18108.x. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Pizzo S. V. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5700–5704. doi: 10.1073/pnas.82.17.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganea D., Teodorescu A., Dray S., Teodorescu M. Polyclonal B-cell activator with esterolytic activity and polyclonal gammopathy induced by allogeneic cells in rabbits. Immunology. 1982 Feb;45(2):227–237. [PMC free article] [PubMed] [Google Scholar]
- Giometti C. S., Anderson N. G., Tollaksen S. L., Edwards J. J., Anderson N. L. Analytical techniques for cell fractions. XXVII. Use of heart proteins as reference standards in two-dimensional electrophoresis. Anal Biochem. 1980 Feb;102(1):47–58. doi: 10.1016/0003-2697(80)90315-2. [DOI] [PubMed] [Google Scholar]
- Got R., Cheftel R. I., Font J., Moretti J. Etude de l'alpha-1-macroglobuline du serum de lapin. 3. Biochimie de la copule glucidique. Biochim Biophys Acta. 1967 Mar 22;136(2):320–330. [PubMed] [Google Scholar]
- Hanover J. A., Willingham M. C., Pastan I. Receptor-mediated endocytosis of alpha 2-macroglobulin: solubilization and partial purification of the fibroblast alpha 2-macroglobulin receptor. Ann N Y Acad Sci. 1983;421:410–423. doi: 10.1111/j.1749-6632.1983.tb18135.x. [DOI] [PubMed] [Google Scholar]
- Harada S., Dannenberg A. M., Jr, Kajiki A., Higuchi K., Tanaka F., Pula P. J. Inflammatory mediators and modulators release in organ culture from rabbit skin lesions produced in vivo by sulfur mustard. II. Evans blue dye experiments that determined the rates of entry and turnover of serum protein in developing and healing lesions. Am J Pathol. 1985 Oct;121(1):28–38. [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Protease inhibitors--a precarious balance. N Engl J Med. 1983 Sep 22;309(12):725–726. doi: 10.1056/NEJM198309223091210. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Keogh E. A. Studies on the physiology of macrophage receptors for alpha-macroglobulin X protease complexes. Ann N Y Acad Sci. 1983;421:442–456. doi: 10.1111/j.1749-6632.1983.tb18138.x. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Dray S. Identification and genetic control of two rabbit alpha 2-macroglobulin allotypes. Biochemistry. 1968 Mar;7(3):1165–1171. doi: 10.1021/bi00843a038. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Abrams W. R., Weinbaum G., Rosenbloom J. Resistance of tropoelastin and elastin peptides to degradation by alpha 2-macroglobulin-protease complexes. Arch Biochem Biophys. 1981 Oct 1;211(1):143–150. doi: 10.1016/0003-9861(81)90439-2. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Lee C. C., Fox R. R., Mills J. K. Genetic heterogeneity of rabbit alpha-1-antitrypsin. Genetics. 1984 Apr;106(4):695–703. doi: 10.1093/genetics/106.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebreton de Vonne T., Mouray H. Isolement d'alpha 1 M et d'alpha-2 M du lapin et leur influence sur l'activité enzymatique de la trypsine. C R Acad Sci Hebd Seances Acad Sci D. 1968 Mar 4;266(10):1076–1079. [PubMed] [Google Scholar]
- Lorand L. Post-translational pathways for generating epsilon(gamma-glutamyl)lysine cross-links. Ann N Y Acad Sci. 1983;421:10–27. doi: 10.1111/j.1749-6632.1983.tb18089.x. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Wing D. A. Synthesis and secretion of alpha2-macroglobulin by cultured human fibroblasts. J Exp Med. 1976 Feb 1;143(2):462–467. doi: 10.1084/jem.143.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Comparison of affinity of trypsin for two -macroglobulin fractions and for 1 -antitrypsin in dog serum. Clin Chim Acta. 1971 Apr;32(2):215–220. doi: 10.1016/0009-8981(71)90335-4. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Ganrot P. O., Laurell C. B. In vivo interaction beween trypsin and some plasma proteins in relation to tolerance to intravenous infusion of trypsin in dog. Acta Chir Scand. 1971;137(2):113–121. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973 Jul 16;36(2):473–481. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Panrucker D. E., Lorscheider F. L. Synthesis of acute-phase alpha 2-macroglobulin during fetal rat development. Ann N Y Acad Sci. 1983;421:391–393. doi: 10.1111/j.1749-6632.1983.tb18130.x. [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Hall P. K. Specificity of proteinases for the "bait" region of alpha 2-macroglobulin. Ann N Y Acad Sci. 1983;421:61–68. doi: 10.1111/j.1749-6632.1983.tb18092.x. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M. The evolution of human alpha 2-macroglobulin: further evidence for a common ancestry with the complement proteins. Ann N Y Acad Sci. 1983;421:112–118. doi: 10.1111/j.1749-6632.1983.tb18097.x. [DOI] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Tamashiro W. K., Powers K. G., Levy D. A., Scott A. L. Quantitative and qualitative changes in the humoral response of dogs through the course of infection with Dirofilaria immitis. Am J Trop Med Hyg. 1985 Mar;34(2):292–301. doi: 10.4269/ajtmh.1985.34.292. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yuan A. I., Feinman R. D. Structure of alpha 2-macroglobulin-protease complexes. Ann N Y Acad Sci. 1983;421:90–97. doi: 10.1111/j.1749-6632.1983.tb18095.x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Aggeler J. Proteases induce secretion of collagenase and plasminogen activator by fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1839–1843. doi: 10.1073/pnas.75.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Reynolds J. J. Stimulation by endocytosis of the secretion of collagenase and neutral proteinase from rabbit synovial fibroblasts. J Exp Med. 1974 Dec 1;140(6):1482–1497. doi: 10.1084/jem.140.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Janoff A., Godfrey H. P. Secretion of Alpha-2-macroglobulin by human alveolar macrophages. Lung. 1980;158(1):9–14. doi: 10.1007/BF02713697. [DOI] [PubMed] [Google Scholar]
- White R., Lee D., Habicht G. S., Janoff A. Secretion of alpha 1-proteinase inhibitor by cultured rat alveolar macrophages. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):447–449. doi: 10.1164/arrd.1981.123.4.447. [DOI] [PubMed] [Google Scholar]
- Wilson G. B., Walker J. H., Jr, Watkins J. H., Jr, Wolgroch D. Determination of subpopulations of leukocytes involved in the synthesis of alpha 1-antitrypsin in vitro. Proc Soc Exp Biol Med. 1980 May;164(1):105–114. doi: 10.3181/00379727-164-40832. [DOI] [PubMed] [Google Scholar]
- van Furth R., Kramps J. A., Diesselhof-den Dulk M. M. Synthesis of alpha 1-anti-trypsin by human monocytes. Clin Exp Immunol. 1983 Mar;51(3):551–557. [PMC free article] [PubMed] [Google Scholar]