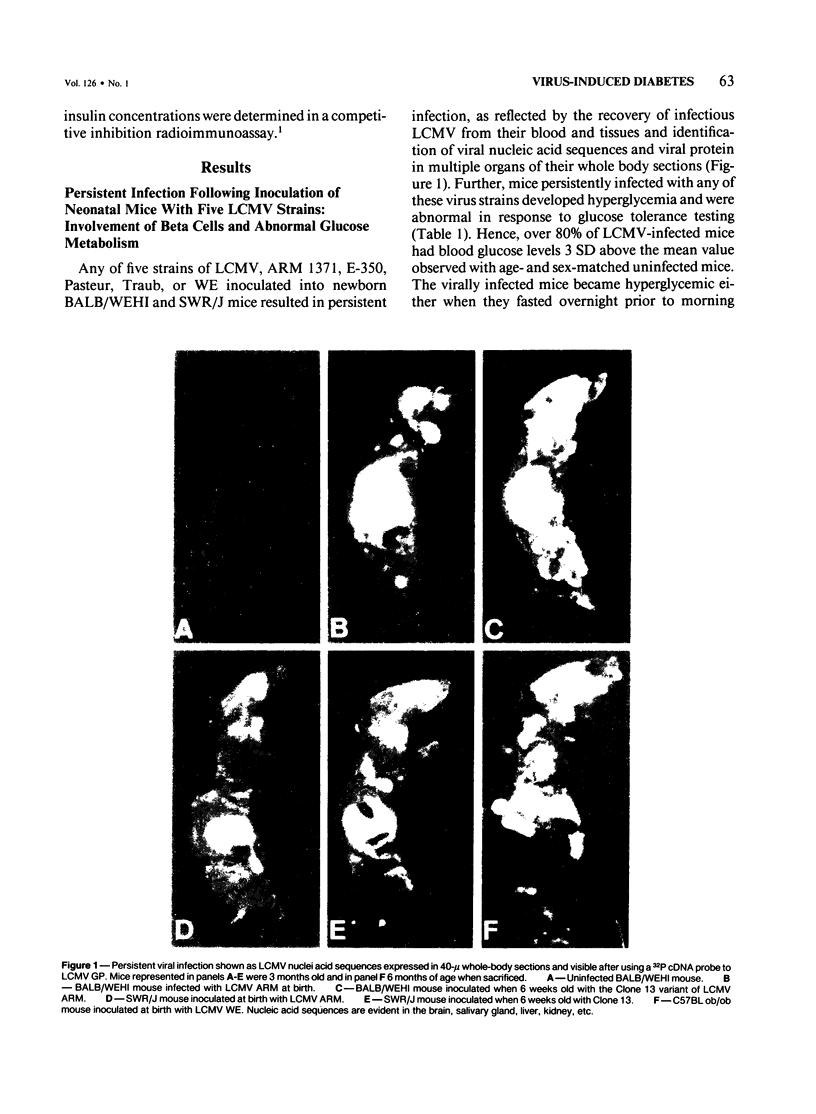
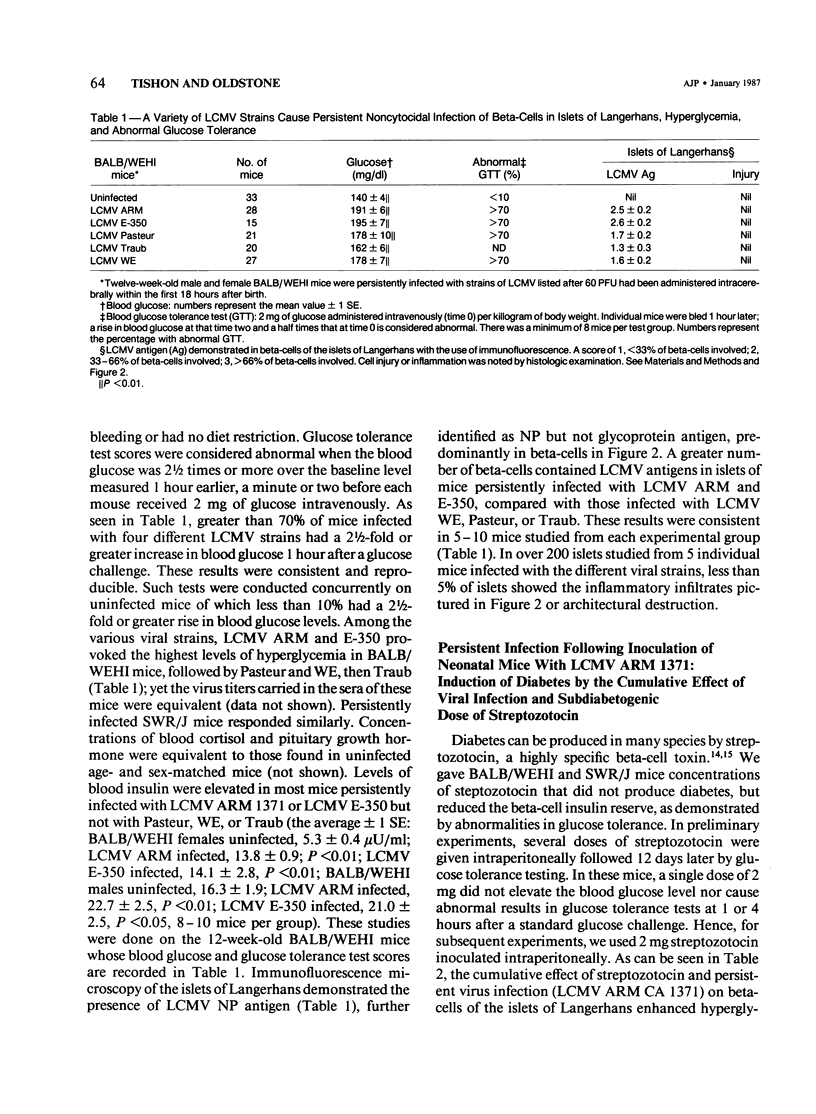
Abstract
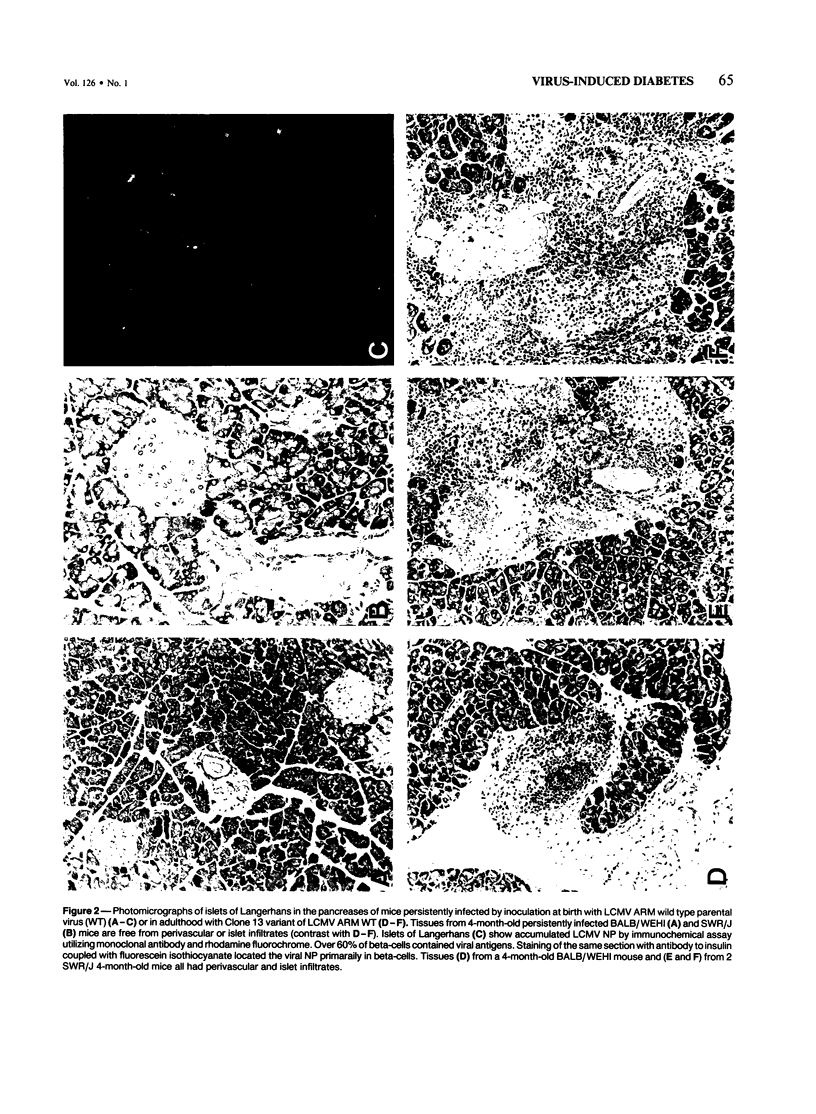
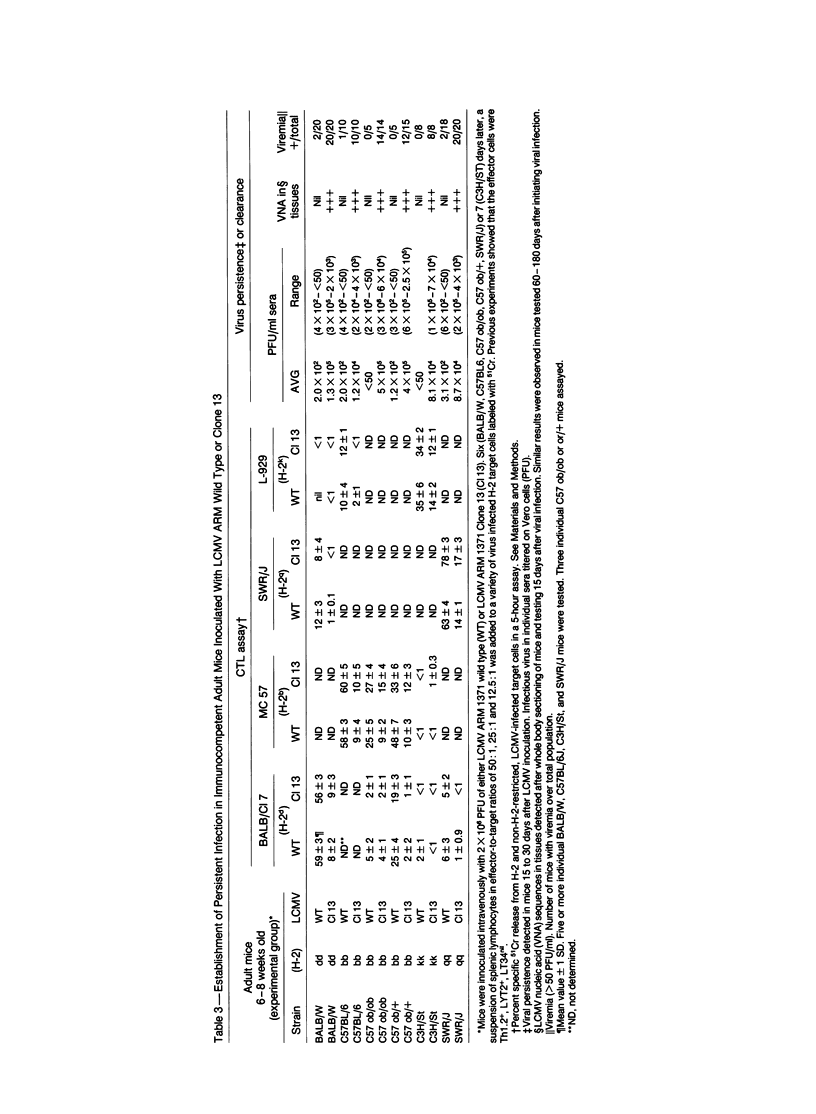
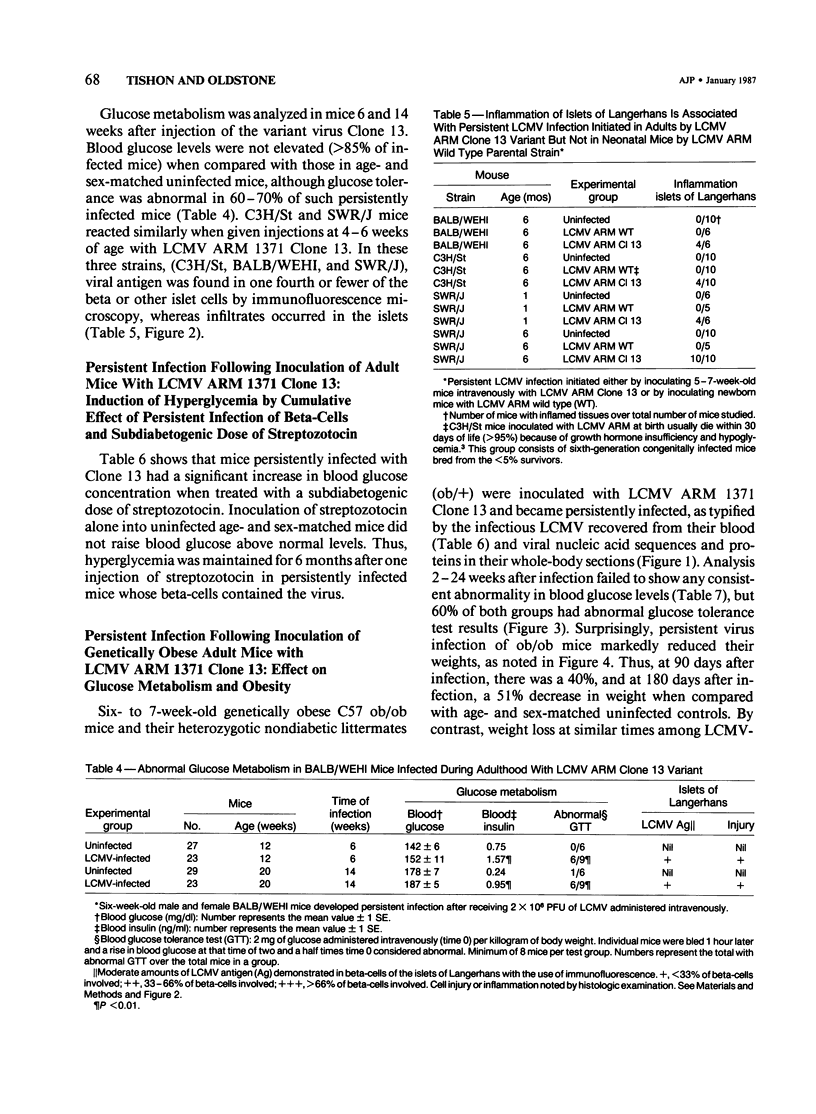
Abnormal glucose metabolism (hyperglycemia and/or aberrant glucose tolerance test) occurred over the life-times of mice persistently infected with lymphocytic choriomeningitis virus (LCMV). Persistent infection could be initiated in both newborn and adult mice. For newborns, inoculation with any of several strains of LCMV (Armstrong, E350, Pasteur, Traub or WE) caused continuous infection, but such infection of adults required a selected lymphotropic variant of LCMV Armstrong (Clone 13). Throughout these animals' lives, viral materials (nucleic acid sequences and proteins) accumulated in multiple tissues, including the beta-cells of the islets of Langerhans; infectious virus was present in blood and tissues, and the LCMV-specific H-2 restricted CTL response was poor. Adult mice that had been inoculated with virus as newborns displayed neither histopathologic injury nor infiltrates of mononuclear cells in the islets of Langerhans despite moderate viral replication in beta-cells. In contrast, mice inoculated as adults with Clone 13 LCMV consistently developed inflammatory infiltrates in perivascular spaces of the islets of Langerhans, and their beta-cells expressed LCMV antigens. Addition of a subdiabetogenic dose of streptozotocin, a specific beta-cell toxin, magnified the virus-induced abnormality in glucose metabolism. This indicated a potentiating role between persistent virus infection initiated at birth or in adulthood and an environmental factor in causing abnormalities in glucose metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Byrne J. A., Oldstone M. B. Virus specificity of cytotoxic T lymphocytes generated during acute lymphocytic choriomeningitis virus infection: role of the H-2 region in determining cross-reactivity for different lymphocytic choriomeningitis virus strains. J Virol. 1984 Jul;51(1):34–41. doi: 10.1128/jvi.51.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton P., Wild T. F., Zwingelstein G. Measles-virus-persistent infection in BGM cells. Modification of the incorporation of [3H]arachidonic acid and [14C]stearic acid into lipids. Biochem J. 1983 Sep 15;214(3):665–670. doi: 10.1042/bj2140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Bernard A., Wild T. F., Tripier M. F. Canine distemper infection in mice: characterization of a neuro-adapted virus strain and its long-term evolution in the mouse. J Gen Virol. 1983 Jul;64(Pt 7):1571–1579. doi: 10.1099/0022-1317-64-7-1571. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Elder J., Lipkin W. I., Southern P. J., Buchmeier M. J., Oldstone M. B. Dissecting the molecular anatomy of the nervous system: analysis of RNA and protein expression in whole body sections of laboratory animals. Brain Res. 1986 Sep 24;382(2):257–265. doi: 10.1016/0006-8993(86)91335-1. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M. J., Faust I. M., Hemmes R. B., Buskirk D. R., Hirsch J., Zabriskie J. B. A virally induced obesity syndrome in mice. Science. 1982 Apr 2;216(4541):82–85. doi: 10.1126/science.7038878. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Ahmed R., Buchmeier M. J., Blount P., Tishon A. Perturbation of differentiated functions during viral infection in vivo. I. Relationship of lymphocytic choriomeningitis virus and host strains to growth hormone deficiency. Virology. 1985 Apr 15;142(1):158–174. doi: 10.1016/0042-6822(85)90430-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Buchmeier M. J. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature. 1982 Nov 25;300(5890):360–362. doi: 10.1038/300360a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Sinha Y. N., Blount P., Tishon A., Rodriguez M., von Wedel R., Lampert P. W. Virus-induced alterations in homeostasis: alteration in differentiated functions of infected cells in vivo. Science. 1982 Dec 10;218(4577):1125–1127. doi: 10.1126/science.7146898. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Southern P., Rodriquez M., Lampert P. Virus persists in beta cells of islets of Langerhans and is associated with chemical manifestations of diabetes. Science. 1984 Jun 29;224(4656):1440–1443. doi: 10.1126/science.6203172. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Oldstone M. B. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J Virol. 1985 Sep;55(3):704–709. doi: 10.1128/jvi.55.3.704-709.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P., Oldstone M. B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985 Apr 15;142(1):175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Southern P. J., Ahmed R., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. V. Recognition is restricted to gene products encoded by the viral S RNA segment. J Immunol. 1986 Jan;136(1):304–307. [PubMed] [Google Scholar]
- Rodriguez M., Garrett R. S., Raitt M., Lampert P. W., Oldstone M. B. Virus persists in beta cells of islets of Langerhans and infection is associated with chemical manifestations of diabetes. II. Morphologic observations. Am J Pathol. 1985 Dec;121(3):497–504. [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Oldstone M. B. Recent clinical isolates of cytomegalovirus suppress human cytomegalovirus-specific human leukocyte antigen-restricted cytotoxic T-lymphocyte activity. J Virol. 1986 Jul;59(1):127–131. doi: 10.1128/jvi.59.1.127-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Blount P., Oldstone M. B. Analysis of persistent virus infections by in situ hybridization to whole-mouse sections. Nature. 1984 Dec 6;312(5994):555–558. doi: 10.1038/312555a0. [DOI] [PubMed] [Google Scholar]
- Toniolo A., Onodera T., Yoon J. W., Notkins A. L. Induction of diabetes by cumulative environmental insults from viruses and chemicals. Nature. 1980 Nov 27;288(5789):383–385. doi: 10.1038/288383a0. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]