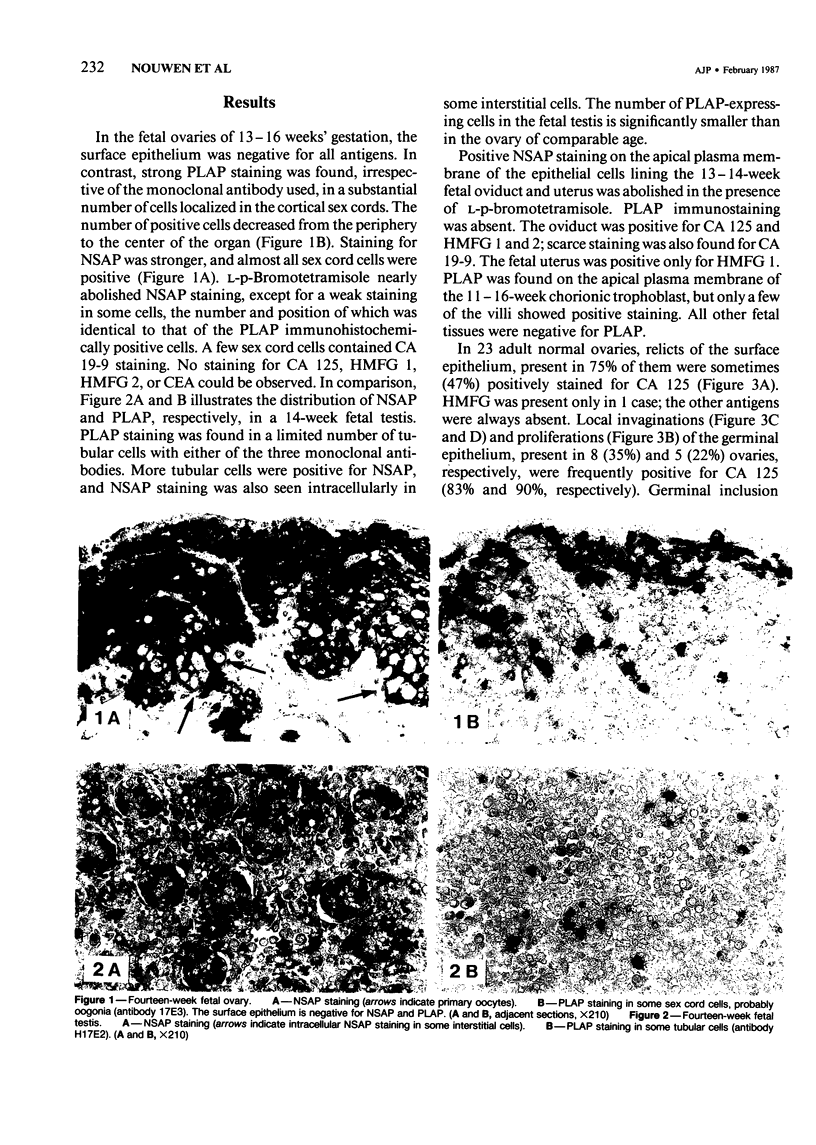
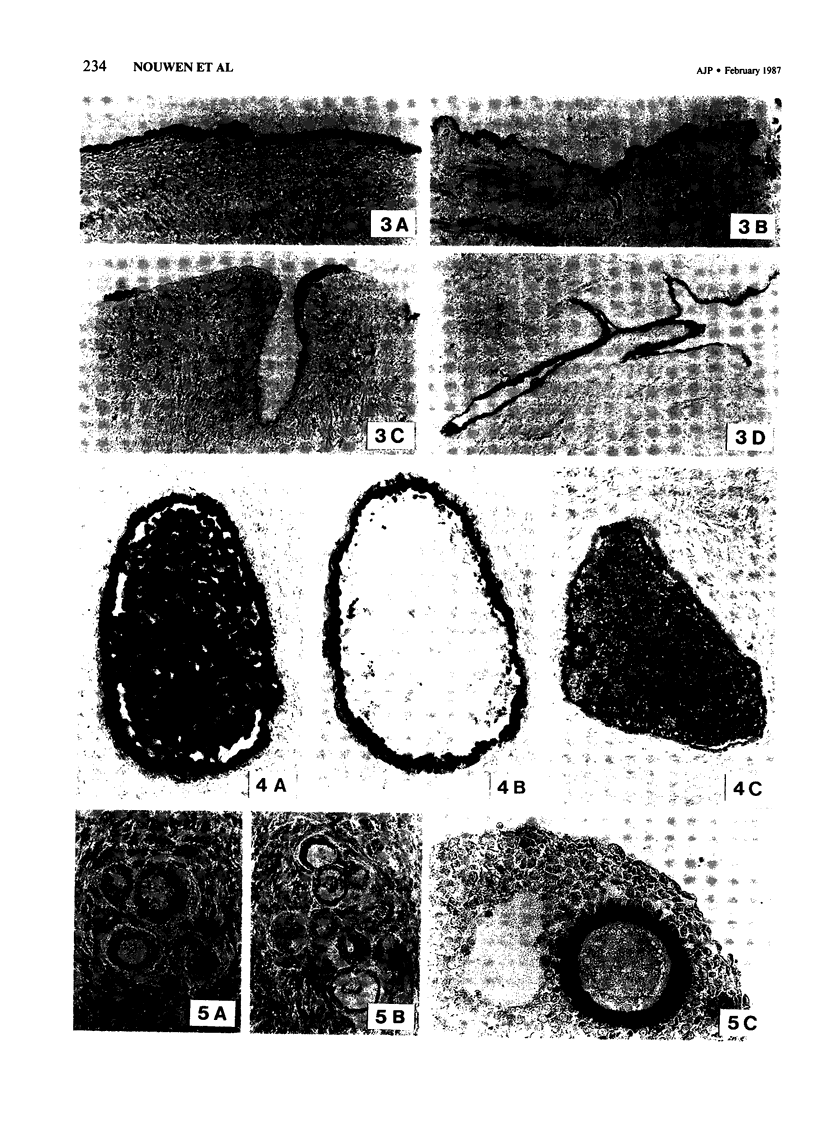
Abstract
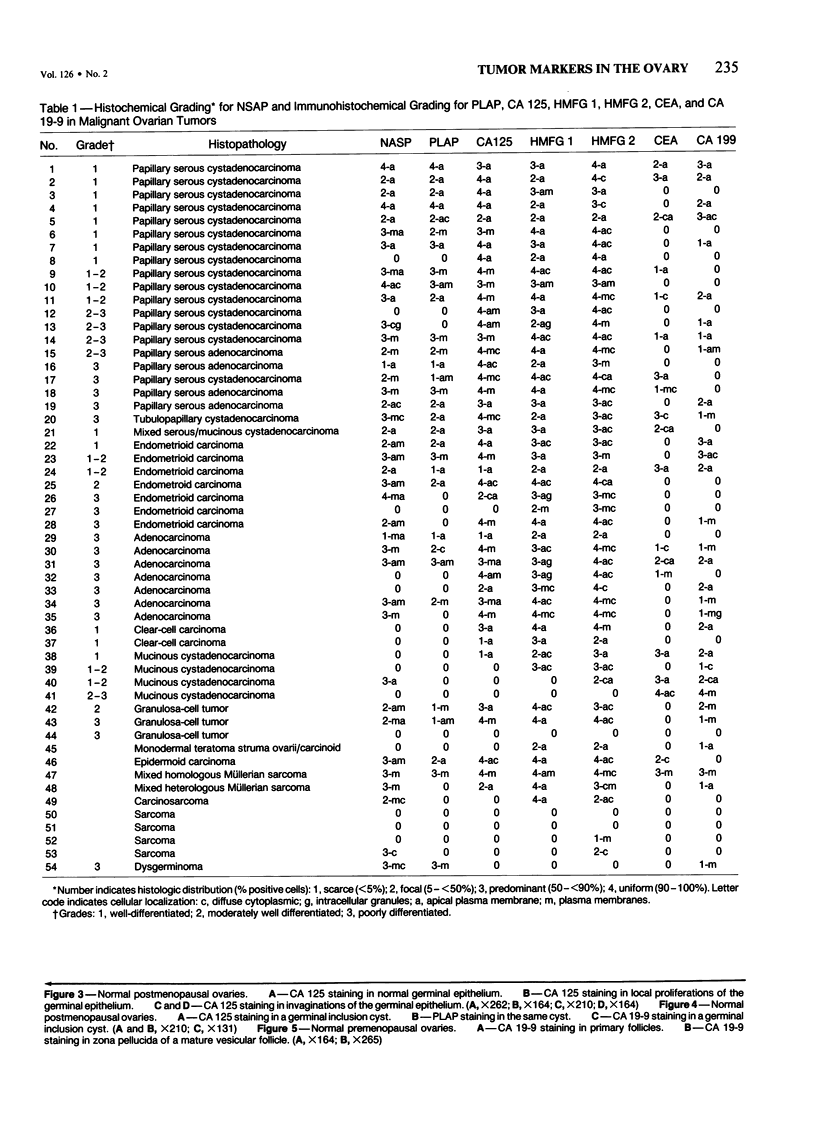
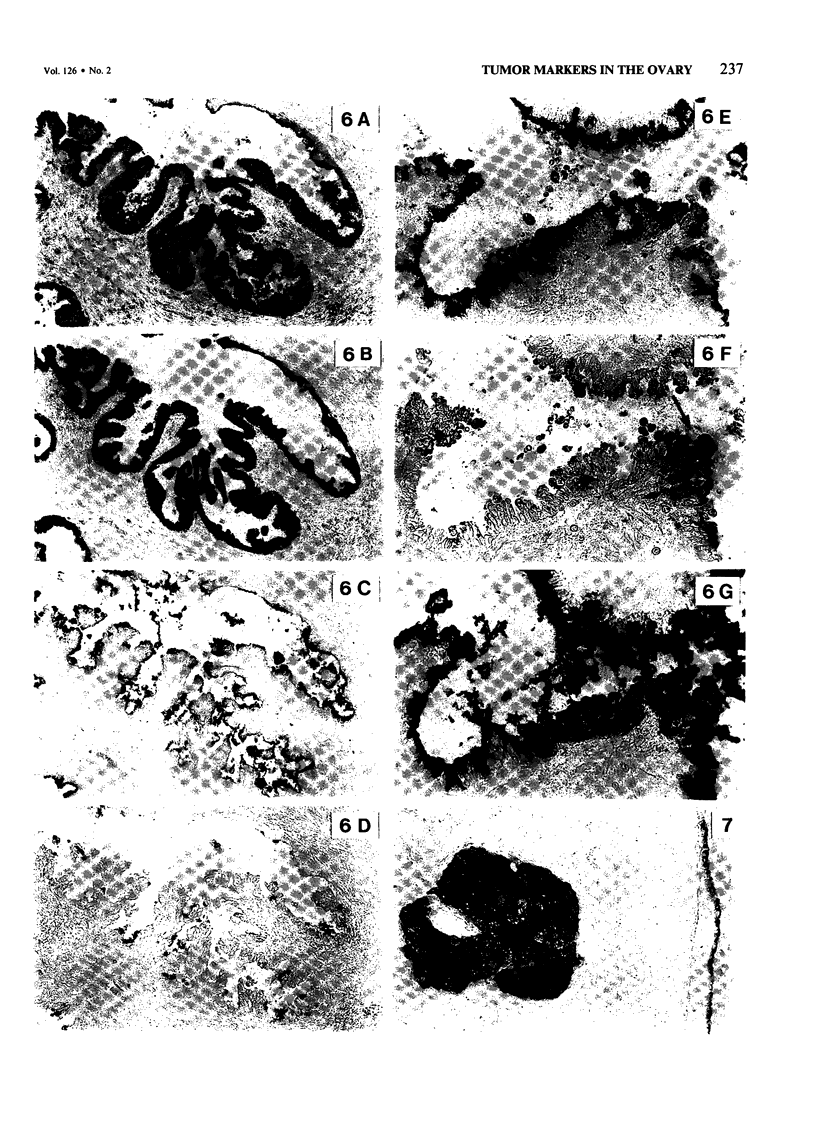
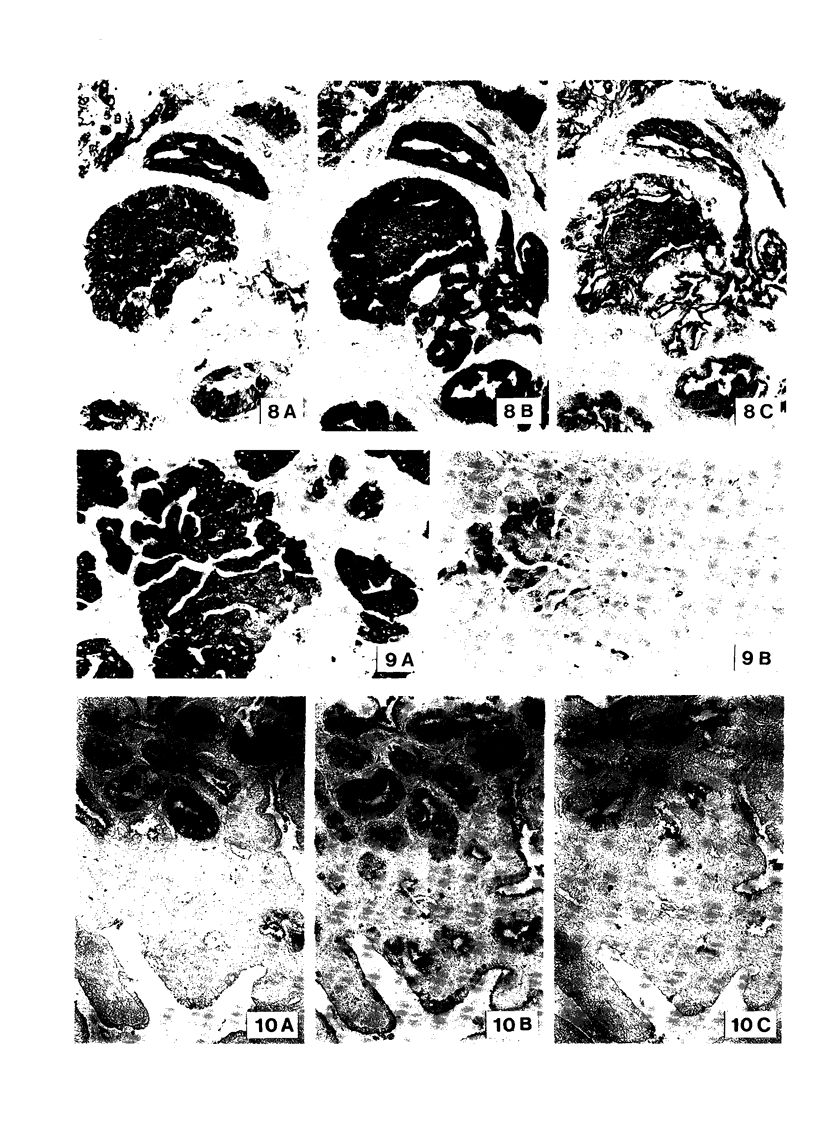
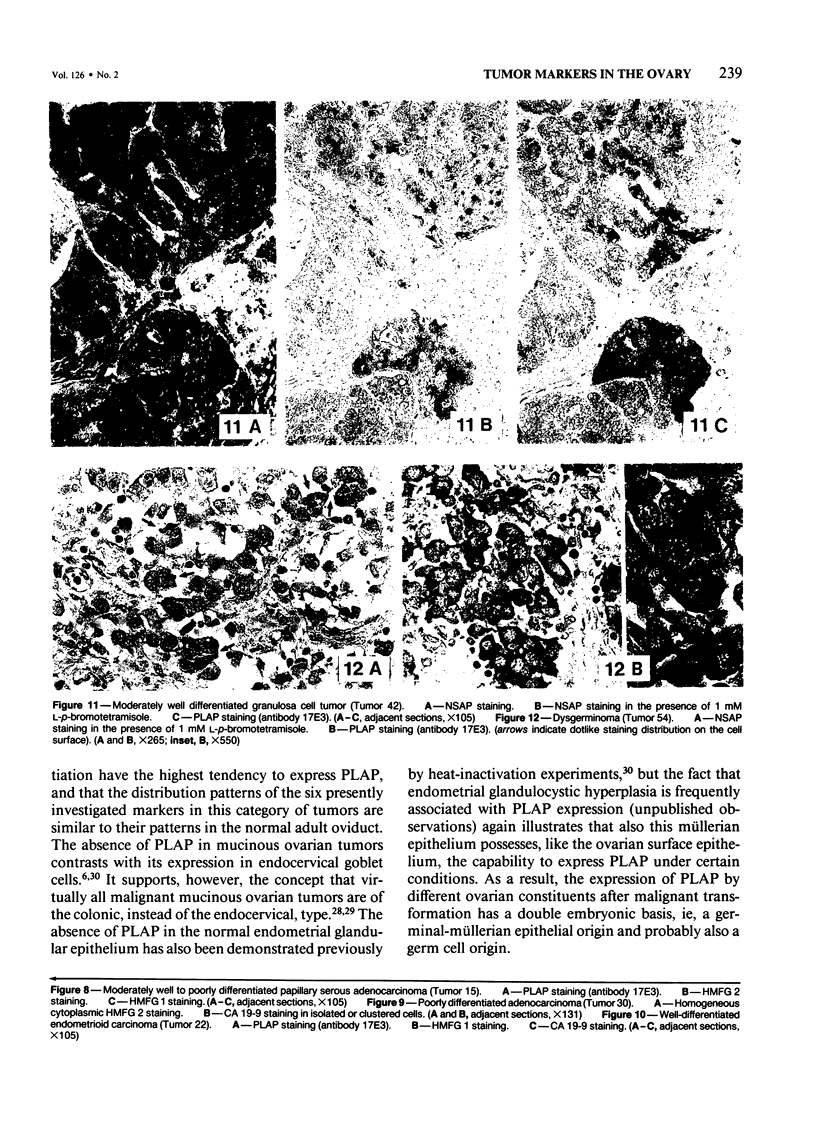
The incidence and histologic characteristics of the expression of placental alkaline phosphatase (PLAP) in ovarian tumors was compared with that of five other tumor antigens. Three monoclonal antibodies were used for the specific localization of PLAP. PLAP was present in some sex cord cells of the 13-16-week fetal ovary, probably germ cells. In normal ovaries, all antigens except carcinoembryonic antigen (CEA) were frequently found in inclusion cysts; the germinal epithelium was positive only for cancer antigen 125 (CA 125). The frequency and extent of PLAP expression in nonmucinous carcinomas was higher than observed for CA 19-9 and CEA, but was lower than for CA 125 and human milk fat globule antigen. Serous tumors had the highest PLAP expression, followed by endometrioid and poorly differentiated adenocarcinomas, and some other tumors. PLAP was predominantly membranous; its histologic distribution was in general heterogeneous. Different antibodies to PLAP gave different staining intensities in some tumors, but the staining patterns were always qualitatively identical.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arklie J., Taylor-Papadimitrious J., Bodmer W., Egan M., Millis R. Differentiation antigens expressed by epithelial cells in the lactating breast are also detectable in breast cancers. Int J Cancer. 1981 Jul 15;28(1):23–29. doi: 10.1002/ijc.2910280105. [DOI] [PubMed] [Google Scholar]
- Atkinson B. F., Ernst C. S., Herlyn M., Steplewski Z., Sears H. F., Koprowski H. Gastrointestinal cancer-associated antigen in immunoperoxidase assay. Cancer Res. 1982 Nov;42(11):4820–4823. [PubMed] [Google Scholar]
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G. On the histogenesis and morphology of ovarian carcinomas. J Cancer Res Clin Oncol. 1984;107(2):71–80. doi: 10.1007/BF00399375. [DOI] [PubMed] [Google Scholar]
- De Groote G., De Waele P., Van de Voorde A., De Broe M., Fiers W. Use of monoclonal antibodies to detect human placental alkaline phosphatase. Clin Chem. 1983 Jan;29(1):115–119. [PubMed] [Google Scholar]
- Edwards P. A. Heterogeneous expression of cell-surface antigens in normal epithelia and their tumours, revealed by monoclonal antibodies. Br J Cancer. 1985 Feb;51(2):149–160. doi: 10.1038/bjc.1985.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerdekens M. W., Nouwen E. J., Pollet D. E., Briers T. W., De Broe M. E. Placental alkaline phosphatase and cancer antigen 125 in sera of patients with benign and malignant diseases. Clin Chem. 1985 May;31(5):687–690. [PubMed] [Google Scholar]
- Epenetos A. A., Britton K. E., Mather S., Shepherd J., Granowska M., Taylor-Papadimitriou J., Nimmon C. C., Durbin H., Hawkins L. R., Malpas J. S. Targeting of iodine-123-labelled tumour-associated monoclonal antibodies to ovarian, breast, and gastrointestinal tumours. Lancet. 1982 Nov 6;2(8306):999–1005. doi: 10.1016/s0140-6736(82)90046-0. [DOI] [PubMed] [Google Scholar]
- Epenetos A. A., Travers P., Gatter K. C., Oliver R. D., Mason D. Y., Bodmer W. F. An immunohistological study of testicular germ cell tumours using two different monoclonal antibodies against placental alkaline phosphatase. Br J Cancer. 1984 Jan;49(1):11–15. doi: 10.1038/bjc.1984.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K. E., Karlsson K. A., Larson G., Thurin J., Blaszczyk M., Steplewski Z., Koprowski H. Mass spectrometry of a human tumor glycolipid antigen being defined by mouse monoclonal antibody NS-19-9. Biochem Biophys Res Commun. 1983 Jan 27;110(2):383–391. doi: 10.1016/0006-291x(83)91160-9. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Blasco L., Harris H. Placental alkaline phosphatase in nonmalignant human cervix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4226–4228. doi: 10.1073/pnas.77.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossrau R. Azoindoxylverfahren zum Hydrolasennachweis. IV. Zur Eignung verschiedener Diazoniumsalze. Histochemistry. 1978 Sep 28;57(4):323–342. doi: 10.1007/BF00492668. [DOI] [PubMed] [Google Scholar]
- Heald J., Buckley C. H., Fox H. An immunohistochemical study of distribution of carcinoembryonic antigen in epithelial tumours of the ovary. J Clin Pathol. 1979 Sep;32(9):918–926. doi: 10.1136/jcp.32.9.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E., Rosenshein N. B., Bhagavan B. S., Parmley T. H. Tumor heterogeneity and histopathology in epithelial ovarian cancer. Obstet Gynecol. 1984 Mar;63(3):330–334. [PubMed] [Google Scholar]
- Hockey M. S., Stokes H. J., Thompson H., Woodhouse C. S., Macdonald F., Fielding J. W., Ford C. H. Carcinoembryonic antigen (CEA) expression and heterogeneity in primary and autologous metastatic gastric tumours demonstrated by a monoclonal antibody. Br J Cancer. 1984 Feb;49(2):129–133. doi: 10.1038/bjc.1984.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Welch W. R., Knapp R. C., Colvin R. B. Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serous, endometrioid, and clear cell types. Am J Clin Pathol. 1983 Jan;79(1):98–104. doi: 10.1093/ajcp/79.1.98. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Kuroki M., Koga Y., Matsuoka Y. Monoclonal antibodies to carcinoembryonic antigen: a systematic analysis of antibody specificities by using related normal antigens and evidence for allotypic determinants on carcinoembryonic antigen. J Immunol. 1984 Oct;133(4):2090–2097. [PubMed] [Google Scholar]
- Malkin A., Kellen J. A., Lickrish G. M., Bush R. S. Carcinoembryonic antigen (CEA) and other tumor markers in ovarian and cervical cancer. Cancer. 1978 Sep;42(3 Suppl):1452–1456. doi: 10.1002/1097-0142(197809)42:3+<1452::aid-cncr2820420813>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- McDicken I. W., McLaughlin P. J., Tromans P. M., Luesley D. M., Johnson P. M. Detection of placental-type alkaline phosphatase in ovarian cancer. Br J Cancer. 1985 Jul;52(1):59–64. doi: 10.1038/bjc.1985.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. R. Intratumor immunologic heterogeneity. Cancer Metastasis Rev. 1982;1(4):319–334. doi: 10.1007/BF00124215. [DOI] [PubMed] [Google Scholar]
- Nouwen E. J., Pollet D. E., Eerdekens M. W., Hendrix P. G., Briers T. W., De Broe M. E. Immunohistochemical localization of placental alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 125 in normal and neoplastic human lung. Cancer Res. 1986 Feb;46(2):866–876. [PubMed] [Google Scholar]
- Nouwen E. J., Pollet D. E., Schelstraete J. B., Eerdekens M. W., Hänsch C., Van de Voorde A., De Broe M. E. Human placental alkaline phosphatase in benign and malignant ovarian neoplasia. Cancer Res. 1985 Feb;45(2):892–902. [PubMed] [Google Scholar]
- Paiva J., Damjanov I., Lange P. H., Harris H. Immunohistochemical localization of placental-like alkaline phosphatase in testis and germ-cell tumors using monoclonal antibodies. Am J Pathol. 1983 May;111(2):156–165. [PMC free article] [PubMed] [Google Scholar]
- Pollet D. E., Nouwen E. J., Schelstraete J. B., Renard J., Van de Voorde A., De Broe M. E. Enzyme-antigen immunoassay for human placental alkaline phosphatase in serum and tissue extracts, and its application as a tumor marker. Clin Chem. 1985 Jan;31(1):41–45. [PubMed] [Google Scholar]
- Russell P. Common epithelial tumours of the ovary--a new look. Pathology. 1985 Oct;17(4):555–557. doi: 10.3109/00313028509084749. [DOI] [PubMed] [Google Scholar]
- Shinoda J., Miwa Y., Sakai N., Yamada H., Shima H., Kato K., Takahashi M., Shimokawa K. Immunohistochemical study of placental alkaline phosphatase in primary intracranial germ-cell tumors. J Neurosurg. 1985 Nov;63(5):733–739. doi: 10.3171/jns.1985.63.5.0733. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., Davies J. O., Stirrat G. M. Immunohistology of normal and ovarian cancer tissue with a monoclonal antibody to placental alkaline phosphatase. Cancer Res. 1984 Oct;44(10):4496–4502. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Travers P., Bodmer W. Preparation and characterization of monoclonal antibodies against placental alkaline phosphatase and other human trophoblast-associated determinants. Int J Cancer. 1984 May 15;33(5):633–641. doi: 10.1002/ijc.2910330514. [DOI] [PubMed] [Google Scholar]
- Van Belle H., De Broe M. E., Wieme R. J. L-p-Bromotetramisole, a new reagent for use in measuring placental or intestinal isoenzymes of alkaline phosphatase in human serum. Clin Chem. 1977 Mar;23(3):454–459. [PubMed] [Google Scholar]
- van Nagell J. R., Jr, Donaldson E. S., Gay E. C., Sharkey R. M., Rayburn P., Goldenberg D. M. Carcinoembryonic antigen in ovarian epithelial cystadenocarcinomas: the prognostic value of tumor and serial plasma determinations. Cancer. 1978 Jun;41(6):2335–2340. doi: 10.1002/1097-0142(197806)41:6<2335::aid-cncr2820410636>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- van de Voorde A., Serreyn R., de Boever J., de Waele P., Vandekerckhove D., Fiers W. The occurrence of human placental alkaline phosphatase (PLAP) in extracts of normal, benign and malignant tissues of the female genital tract. Tumour Biol. 1985;6(6):545–553. [PubMed] [Google Scholar]