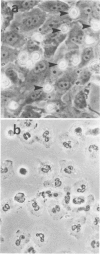
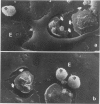
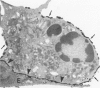
Abstract
Polymorphonuclear leukocytes can produce active oxygen species such as hydrogen peroxide and superoxide under various conditions. Because these substances can be toxic to cells, it is possible that the interaction between the circulating leukocytes and the blood vessel wall, either in normal circulation or during the acute inflammatory response, could damage the endothelial lining. Using an in vitro system of cultured endothelial cells and isolated polymorphonuclear leukocytes, we have measured the levels of detectable superoxide when neutrophils are attached to either endothelial monolayers or to plastic. Our results show that the levels of superoxide, on a per-cell basis, are lower when the neutrophils are attached to endothelium than when attached to plastic, even if the neutrophils are stimulated with phorbol myristate acetate. This is also reflected in data showing that no injury occurs to the endothelial cells, as measured by 51Cr release, under these same conditions. When endothelial cells are pretreated with an inhibitor of superoxide dismutase, diethyldithiocarbamate, the levels of superoxide detected are the same for neutrophils stimulated on plastic and those on the endothelial monolayer, suggesting that endothelial superoxide dismutase may remove a portion of the neutrophil-generated superoxide from the detection system. Further evidence for the role of endothelium in destroying superoxide is suggested by results that show that the level of detectable superoxide released from neutrophils attached to formalin-fixed endothelial monolayers is the same as that for neutrophils attached to plastic. It is important to note that with the inhibitor of superoxide dismutase present, the endothelial monolayers do not display enhanced 51Cr release under the conditions employed. When both endothelial catalase and glutathione reductase are inhibited, we detect increased 51Cr release from endothelial cells in response to stimulated neutrophils. Our results show that the endothelial cells are important in affecting the apparent reduction of toxic oxygen products derived from polymorphonuclear leukocytes attached to their surface.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvasc Res. 1981 Nov;22(3):239–254. doi: 10.1016/0026-2862(81)90095-9. [DOI] [PubMed] [Google Scholar]
- Fehr J., Moser R., Leppert D., Groscurth P. Antiadhesive properties of biological surfaces are protective against stimulated granulocytes. J Clin Invest. 1985 Aug;76(2):535–542. doi: 10.1172/JCI112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes R. D., Guttmann R. D. Evidence for complement-induced endothelial injury in vivo: a comparative ultrastructural tracer study in a controlled model of hyperacute rat cardiac allograft rejection. Am J Pathol. 1982 Mar;106(3):378–387. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Hoffstein S. T., Gennaro D. E., Manzi R. M. Surface contact inhibits neutrophil superoxide generation induced by soluble stimuli. Lab Invest. 1985 May;52(5):515–522. [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute and progressive lung injury after contact with phorbol myristate acetate. Am J Pathol. 1982 Apr;107(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. Receptor-mediated regulation of superoxide production in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1981 Nov;68(5):1314–1320. doi: 10.1172/JCI110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Arfors K. E. Changes in macromolecular permeability by intravascular generation of oxygen-derived free radicals. Microvasc Res. 1982 Jul;24(1):25–33. doi: 10.1016/0026-2862(82)90039-5. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Hoffman L. H., Brigham K. L. Chemotaxis of granulocytes across bovine pulmonary artery intimal explants without endothelial cell injury. Tissue Cell. 1984;16(1):1–16. doi: 10.1016/0040-8166(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Cohn Z. A. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981 Nov 1;154(5):1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Roos D. S., Davidson R. L., Karnovsky M. J. Membrane alterations and other morphological features associated with polyethylene glycol-induced cell fusion. J Cell Sci. 1979 Dec;40:63–75. doi: 10.1242/jcs.40.1.63. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Peach M. J. Granulocytes and phorbol myristate acetate increase permeability to albumin of cultured endothelial monolayers and isolated perfused lungs. Role of oxygen radicals and granulocyte adherence. Am Rev Respir Dis. 1983 Jan;127(1):72–76. doi: 10.1164/arrd.1983.127.1.72. [DOI] [PubMed] [Google Scholar]
- Steinberg H., Greenwald R. A., Sciubba J., Das D. K. The effect of oxygen-derived free radicals on pulmonary endothelial cell function in the isolated perfused rat lung. Exp Lung Res. 1982 May;3(2):163–173. doi: 10.3109/01902148209063290. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Ritchie W. G., Lynch P. R. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974 Mar;74(3):507–532. [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Taub M., Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada O., Moldow C. F., Sacks T., Craddock P. R., Boogaerts M. A., Jacob H. S. Deleterious effects of endotoxin on cultured endothelial cells: an in vitro model of vascular injury. Inflammation. 1981 Jun;5(2):115–126. doi: 10.1007/BF00914201. [DOI] [PubMed] [Google Scholar]