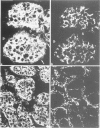
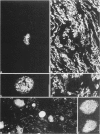
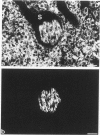
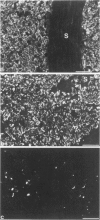
Abstract
Synaptophysin is an integral membrane glycoprotein originally isolated from presynaptic vesicles of bovine neurons. The authors have studied a wide spectrum of neuroendocrine (NE) neoplasms by immunofluorescence microscopy on cryostat sections of freshly frozen tissues using a monoclonal antibody to this protein (SY 38). Without exception, they found the identical--or a very similar--protein expressed in all neuroblastomas, ganglioneuroblastomas, ganglioneuromas, pheochromocytomas, and paragangliomas studied. In these "neural" type NE neoplasms, synaptophysin was coexpressed with neurofilament proteins. Synaptophysin was also demonstrated in NE neoplasms of "epithelial" type in which it was predominantly coexpressed with cytokeratins and desmoplakin. It was invariably found in all variants of islet cell neoplasms and in all medullary thyroid carcinomas. Synaptophysin was also demonstrated in several adenomas of the hypophysis and parathyroids, in the majority of carcinoids of the bronchopulmonary and gastrointestinal tracts, and in many, though not all, NE carcinomas of the same sites, and of the skin. Conversely, SY 38 did not immunostain any of a large number of benign and malignant non-NE epithelial neoplasms; nor was any immunostaining obtained in a group of mesenchymal tumors. It is remarkable that SY 38 did not immunostain a number of malignant melanomas, including several that were immunostained for neuron-specific enolase (NSE) and several neuropeptides. Parallel studies conducted on conventionally fixed, paraffin-embedded tissue sections immunostained by the use of the avidin-biotin complex technique yielded very similar results. The findings indicate that synaptophysin is expressed in the whole range of NE neoplasms without detectable relation to the expression of other NE markers such as NSE, serotonin, and neuropeptides. Nor could the expression of synaptophysin by these tumors be correlated with their epithelial and/or neural cytoskeletal characteristics, their clinical aggressiveness, or the presence or absence of endocrinologic abnormalities. While the consistent expression of synaptophysin by the "neural" type of NE neoplasms would seem predictable its presence in diverse benign and malignant NE tumors of "epithelial" type is remarkable. It is concluded that synaptophysin is a significant as well as novel NE marker, and the use of antibody SY 38 as a broad range marker for the study and diagnosis of NE neoplasms is proposed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew A. An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embryol Exp Morphol. 1976 Jun;35(3):577–593. [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Facer P., Ferri G. L., Marangos P. J., Pearse A. G. Neuron specific enolase: a common marker for the endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982 Oct;83(4):902–915. [PubMed] [Google Scholar]
- Blobel G. A., Gould V. E., Moll R., Lee I., Huszar M., Geiger B., Franke W. W. Coexpression of neuroendocrine markers and epithelial cytoskeletal proteins in bronchopulmonary neuroendocrine neoplasms. Lab Invest. 1985 Jan;52(1):39–51. [PubMed] [Google Scholar]
- Broers J. L., Carney D. N., de Ley L., Vooijs G. P., Ramaekers F. C. Differential expression of intermediate filament proteins distinguishes classic from variant small-cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4409–4413. doi: 10.1073/pnas.82.13.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. V., Zangerle R., Fischer-Colbrie R., Chu L. L., Elting J. J., Hamilton J. W., Winkler H. Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6056–6059. doi: 10.1073/pnas.79.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Levy R., Franke W. W. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol. 1985 May;37:175–190. [PubMed] [Google Scholar]
- Erlandson R. A., Woodruff J. M. Peripheral nerve sheath tumors: an electron microscopic study of 43 cases. Cancer. 1982 Jan 15;49(2):273–287. doi: 10.1002/1097-0142(19820115)49:2<273::aid-cncr2820490213>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Gigi O., Geiger B., Eshhar Z., Moll R., Schmid E., Winter S., Schiller D. L., Franke W. W. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. doi: 10.1002/j.1460-2075.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Linnoila R. I., Memoli V. A., Warren W. H. Neuroendocrine components of the bronchopulmonary tract: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1983 Nov;49(5):519–537. [PubMed] [Google Scholar]
- Gould V. E., Memoli V., Chejfec G., Johannessen J. V. The APUD cell system and its neoplasms: observations on the significance and limitations of the concept. Surg Clin North Am. 1979 Feb;59(1):93–108. doi: 10.1016/s0039-6109(16)41736-6. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Moll R., Moll I., Lee I., Franke W. W. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985 Apr;52(4):334–353. [PubMed] [Google Scholar]
- Haimoto H., Takahashi Y., Koshikawa T., Nagura H., Kato K. Immunohistochemical localization of gamma-enolase in normal human tissues other than nervous and neuroendocrine tissues. Lab Invest. 1985 Mar;52(3):257–263. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Mervak T., Schmidt K., Warner T. F., Wilson B. S. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. Am J Surg Pathol. 1984 Aug;8(8):607–614. doi: 10.1097/00000478-198408000-00004. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Clark R., Lehto V. P., Virtanen I., Damjanov I. Intermediate-filament proteins in parathyroid glands and parathyroid adenomas. Arch Pathol Lab Med. 1985 Nov;109(11):986–989. [PubMed] [Google Scholar]
- Miettinen M., Franssila K., Lehto V. P., Paasivuo R., Virtanen I. Expression of intermediate filament proteins in thyroid gland and thyroid tumors. Lab Invest. 1984 Mar;50(3):262–270. [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Dahl D., Virtanen I. Varying expression of cytokeratin and neurofilaments in neuroendocrine tumors of human gastrointestinal tract. Lab Invest. 1985 Apr;52(4):429–436. [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Immunofluorescence microscopic evaluation of the intermediate filament expression of the adrenal cortex and medulla and their tumors. Am J Pathol. 1985 Mar;118(3):360–366. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Moll R., Moll I., Franke W. W. Identification of Merkel cells in human skin by specific cytokeratin antibodies: changes of cell density and distribution in fetal and adult plantar epidermis. Differentiation. 1984;28(2):136–154. doi: 10.1111/j.1432-0436.1984.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Mukai M., Torikata C., Iri H., Morikawa Y., Shimizu K., Shimoda T., Nukina N., Ihara Y., Kageyama K. Expression of neurofilament triplet proteins in human neural tumors. An immunohistochemical study of paraganglioma, ganglioneuroma, ganglioneuroblastoma, and neuroblastoma. Am J Pathol. 1986 Jan;122(1):28–35. [PMC free article] [PubMed] [Google Scholar]
- Nesland J. M., Holm R., Johannessen J. V., Gould V. E. Neurone specific enolase immunostaining in the diagnosis of breast carcinomas with neuroendocrine differentiation. Its usefulness and limitations. J Pathol. 1986 Jan;148(1):35–43. doi: 10.1002/path.1711480107. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Immunoreactive human chromogranin A in diverse polypeptide hormone producing human tumors and normal endocrine tissues. J Clin Endocrinol Metab. 1983 Nov;57(5):1084–1086. doi: 10.1210/jcem-57-5-1084. [DOI] [PubMed] [Google Scholar]
- Osborn M., Dirk T., Käser H., Weber K., Altmannsberger M. Immunohistochemical localization of neurofilaments and neuron-specific enolase in 29 cases of neuroblastoma. Am J Pathol. 1986 Mar;122(3):433–442. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pearse A. G., Takor T. Embryology of the diffuse neuroendocrine system and its relationship to the common peptides. Fed Proc. 1979 Aug;38(9):2288–2294. [PubMed] [Google Scholar]
- Pearse A. G. The APUD cell concept and its implications in pathology. Pathol Annu. 1974;9(0):27–41. [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The diffuse neuroendocrine system and the apud concept: related "endocrine" peptides in brain, intestine, pituitary, placenta, and anuran cutaneous glands. Med Biol. 1977 Jun;55(3):115–125. [PubMed] [Google Scholar]
- Pictet R. L., Rall L. B., Phelps P., Rutter W. J. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976 Jan 16;191(4223):191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Esscher T., Nilsson K. Expression of gamma-subunit of enolase, neuron-specific enolase, in human non-neuroendocrine tumors and derived cell lines. Lab Invest. 1986 May;54(5):554–560. [PubMed] [Google Scholar]
- Rehm H., Wiedenmann B., Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO J. 1986 Mar;5(3):535–541. doi: 10.1002/j.1460-2075.1986.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel D. E. Gamma-subunit of the glycolytic enzyme enolase: nonspecific or neuron specific? Lab Invest. 1985 Mar;52(3):239–242. [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978 Dec 21;276(5690):834–836. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]
- Schröder S., Dockhorn-Dworniczak B., Kastendieck H., Böcker W., Franke W. W. Intermediate-filament expression in thyroid gland carcinomas. Virchows Arch A Pathol Anat Histopathol. 1986;409(6):751–766. doi: 10.1007/BF00710761. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., DePotter R. W., Fischer-Colbrie R., Schober M., Winkler H., Chubb I. W. Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res. 1984 Dec;320(2-3):193–230. doi: 10.1016/0165-0173(84)90007-9. [DOI] [PubMed] [Google Scholar]
- Tapia F. J., Polak J. M., Barbosa A. J., Bloom S. R., Marangos P. J., Dermody C., Pearse A. G. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981 Apr 11;1(8224):808–811. doi: 10.1016/s0140-6736(81)92682-9. [DOI] [PubMed] [Google Scholar]
- Warren W. H., Memoli V. A., Gould V. E. Immunohistochemical and ultrastructural analysis of bronchopulmonary neuroendocrine neoplasms. I. Carcinoids. Ultrastruct Pathol. 1984;6(1):15–27. doi: 10.3109/01913128409016661. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W., Kuhn C., Moll R., Gould V. E. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A. 1986 May;83(10):3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]