Abstract
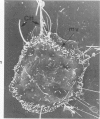
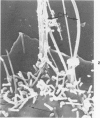
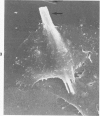
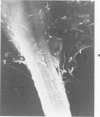
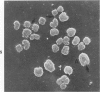
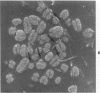
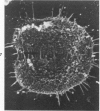
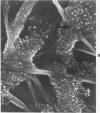
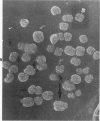
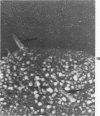
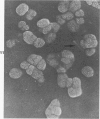
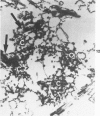
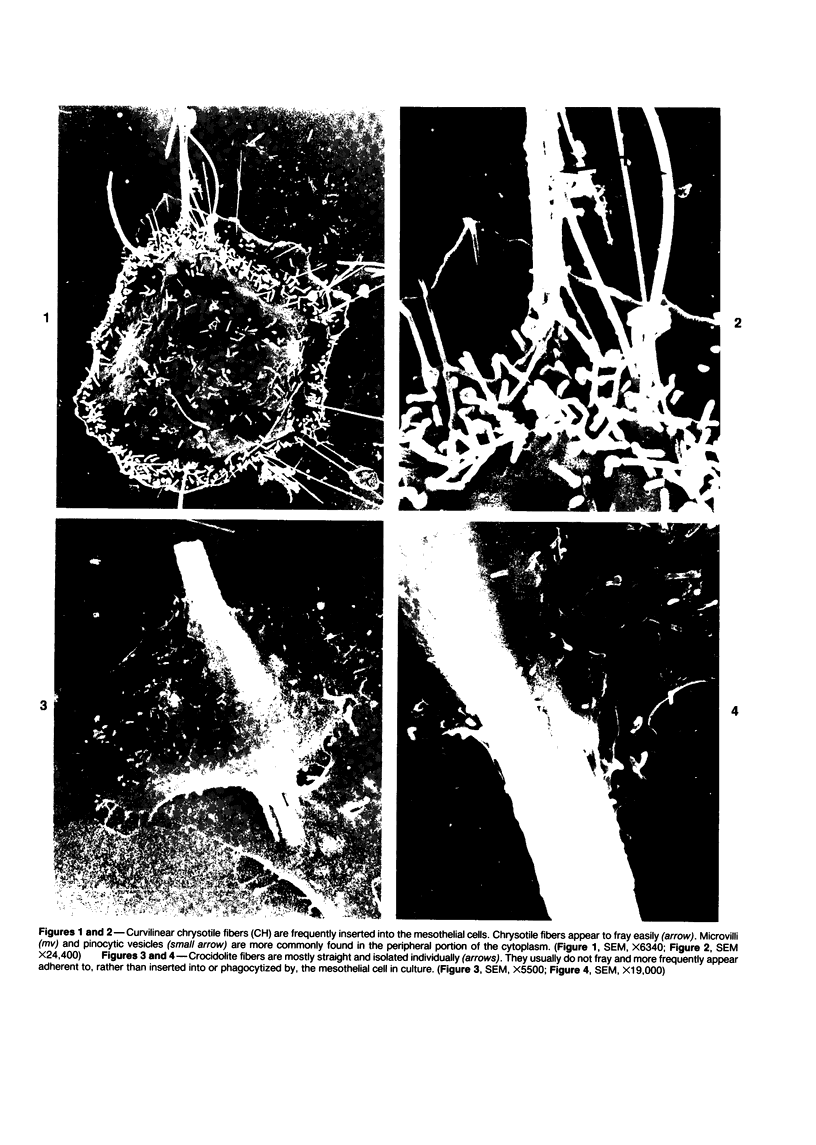
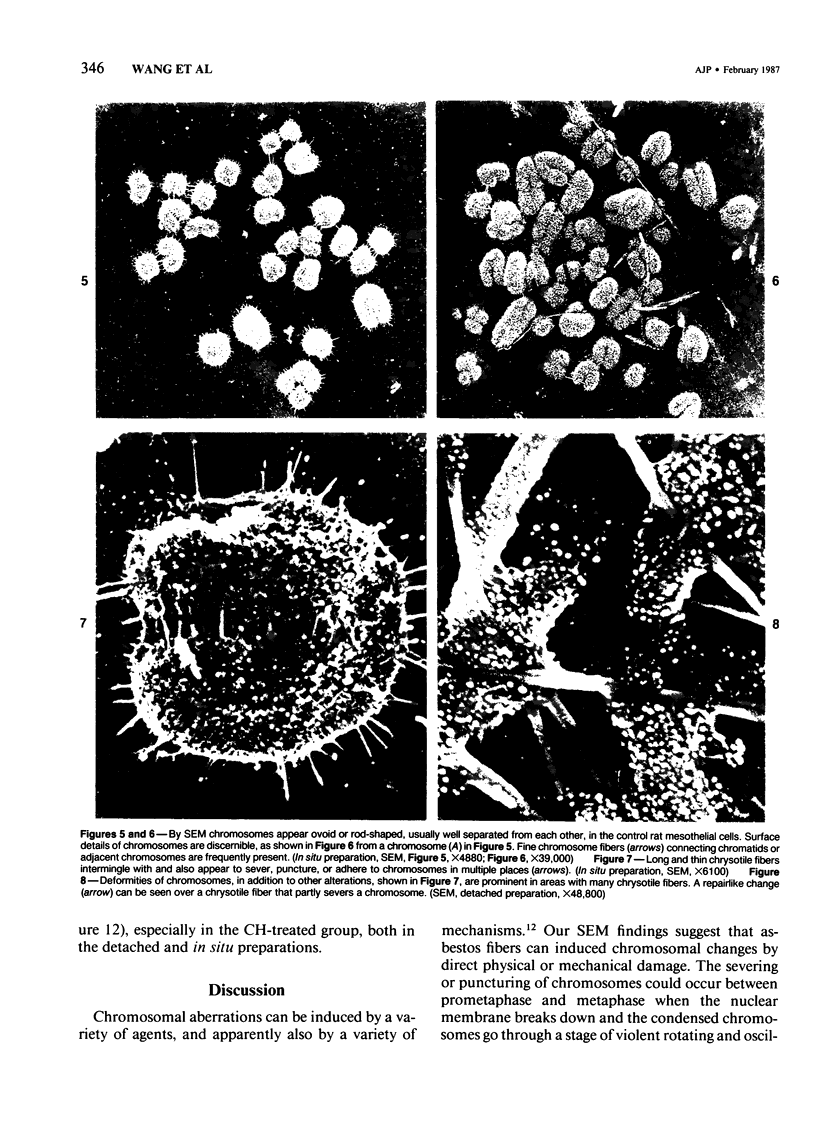
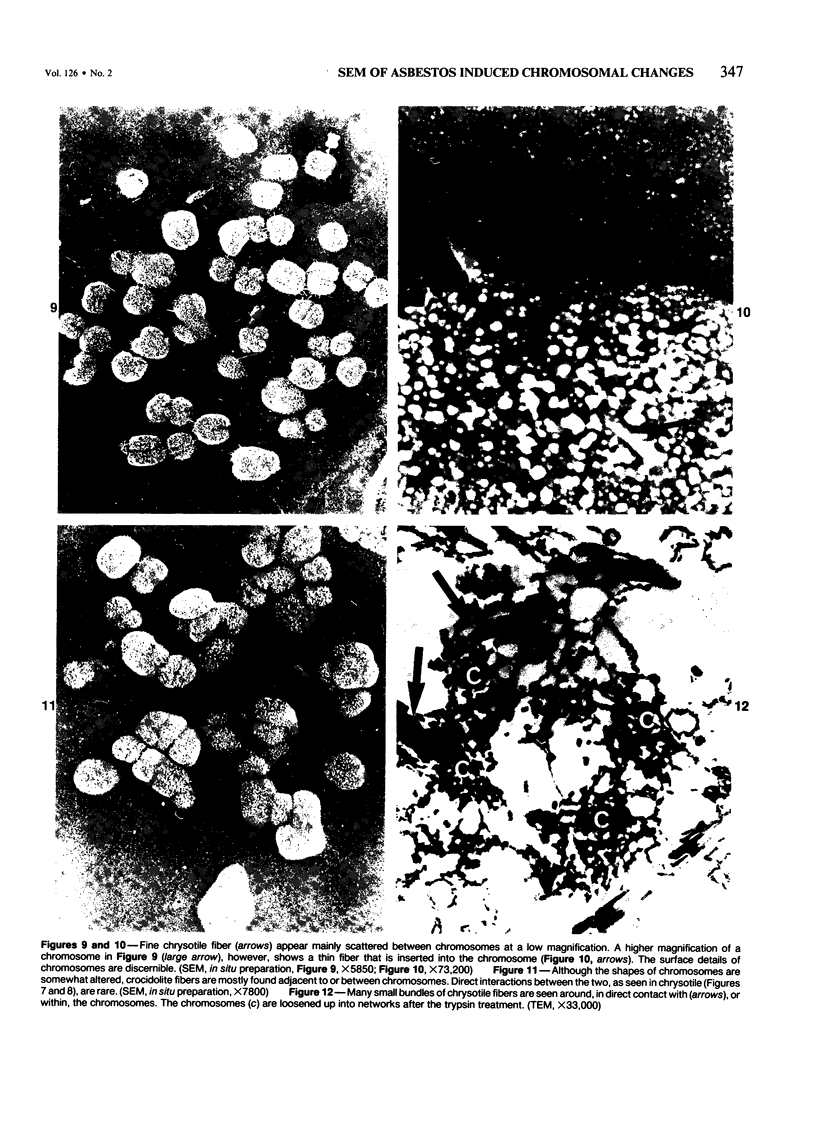
Rat pleural mesothelial cells (PMCs) in culture at the exponential growing phase were exposed to 5 micrograms/ml of chrysotile (CH) or crocidolite (CR) asbestos fibers: the cells and their chromosomes were studied 48 hours thereafter by light, scanning, and transmission electron microscopy (LM, SEM, TEM). PMCs phagocytized both CH and CR. Mild vacuolar cytoplasmic changes by LM and a few small surface blebbings by SEM were present, mainly in cells treated with CH. Metaphase chromosomes were well separated and retained surface details by SEM in the control group. Chromosomes were frequently entangled with, adherent to, and severed or pierced by long and thin curvilinear CH with occasional chromatin fibers threading over the partly severed asbestos. Similar chromosomal changes were much less frequently found in CR-treated cells; TEM confirmed the same findings. CH and CR have different physicochemical properties and also appear to have direct, intricate, but different interactions with chromosomes, as well as the cytoplasm, of PMCs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg D. A., Ellis G. W. Micromanipulation studies of chromosome movement. II. Birefringent chromosomal fibers and the mechanical attachment of chromosomes to the spindle. J Cell Biol. 1979 Aug;82(2):542–554. doi: 10.1083/jcb.82.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. F., Ford J. O. Early response of the visceral pleura following asbestos exposure: an ultrastructural study. J Toxicol Environ Health. 1985;15(5):673–686. doi: 10.1080/15287398509530695. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Allison A. C., Badami D. V. Mineral fibers: chemical, physicochemical, and biological properties. Adv Pharmacol Chemother. 1975;12(0):291–402. doi: 10.1016/s1054-3589(08)60223-9. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Miller K., Macnab G. Hemolysis by asbestos. Environ Res. 1971 Apr;4(2):95–117. doi: 10.1016/0013-9351(71)90038-7. [DOI] [PubMed] [Google Scholar]
- Harrison C. J., Allen T. D., Britch M., Harris R. High-resolution scanning electron microscopy of human metaphase chromosomes. J Cell Sci. 1982 Aug;56:409–422. doi: 10.1242/jcs.56.1.409. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985 Mar;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Bastie-Sigeac I., Bignon J., Stoebner P. Effect of chrysotile and crocidolite on the morphology and growth of rat pleural mesothelial cells. Environ Res. 1983 Apr;30(2):255–269. doi: 10.1016/0013-9351(83)90212-8. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Kheuang L., Magne L., Bignon J. Chromosomal changes induced by chrysotile fibres or benzo-3,4-pyrene in rat pleural mesothelial cells. Mutat Res. 1986 Mar;169(3):141–148. doi: 10.1016/0165-1218(86)90093-5. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Pooley F. D., Smith P. G. Factory populations exposed to crocidolite asbestos - a continuing survey. IARC Sci Publ. 1976;(13):117–120. [PubMed] [Google Scholar]
- McDonald J. C., Liddell F. D., Gibbs G. W., Eyssen G. E., McDonald A. D. Dust exposure and mortality in chrysotile mining, 1910-75. Br J Ind Med. 1980 Feb;37(1):11–24. doi: 10.1136/oem.37.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman B., Light W., Wei E. Asbestos: mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu Rev Pharmacol Toxicol. 1983;23:595–615. doi: 10.1146/annurev.pa.23.040183.003115. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Hesterberg T. W., Tsutsui T., Barrett J. C. Correlation of asbestos-induced cytogenetic effects with cell transformation of Syrian hamster embryo cells in culture. Cancer Res. 1984 Nov;44(11):5017–5022. [PubMed] [Google Scholar]
- Paterour M. J., Bignon J., Jaurand M. C. In vitro transformation of rat pleural mesothelial cells by chrysotile fibres and/or benzo[a]pyrene. Carcinogenesis. 1985 Apr;6(4):523–529. doi: 10.1093/carcin/6.4.523. [DOI] [PubMed] [Google Scholar]
- Sincock A., Seabright M. Induction of chromosome changes in Chinese hamster cells by exposure to asbestos fibres. Nature. 1975 Sep 4;257(5521):56–58. doi: 10.1038/257056a0. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 1972 Mar;48(3):797–821. [PubMed] [Google Scholar]
- Topping D. C., Nettesheim P. Two-stage carcinogenesis studies with asbestos in Fischer 344 rats. J Natl Cancer Inst. 1980 Sep;65(3):627–630. [PubMed] [Google Scholar]
- WAGNER J. C., SLEGGS C. A., MARCHAND P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960 Oct;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C., Berry G. Mesotheliomas in rats following inoculation with asbestos. Br J Cancer. 1969 Sep;23(3):567–581. doi: 10.1038/bjc.1969.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. S. The surface appearance of some lung tumors, mesothelioma and their precursor lesions. Scan Electron Microsc. 1980;(3):79–88. [PubMed] [Google Scholar]
- Weitzman S. A., Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch Biochem Biophys. 1984 Jan;228(1):373–376. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]