Abstract
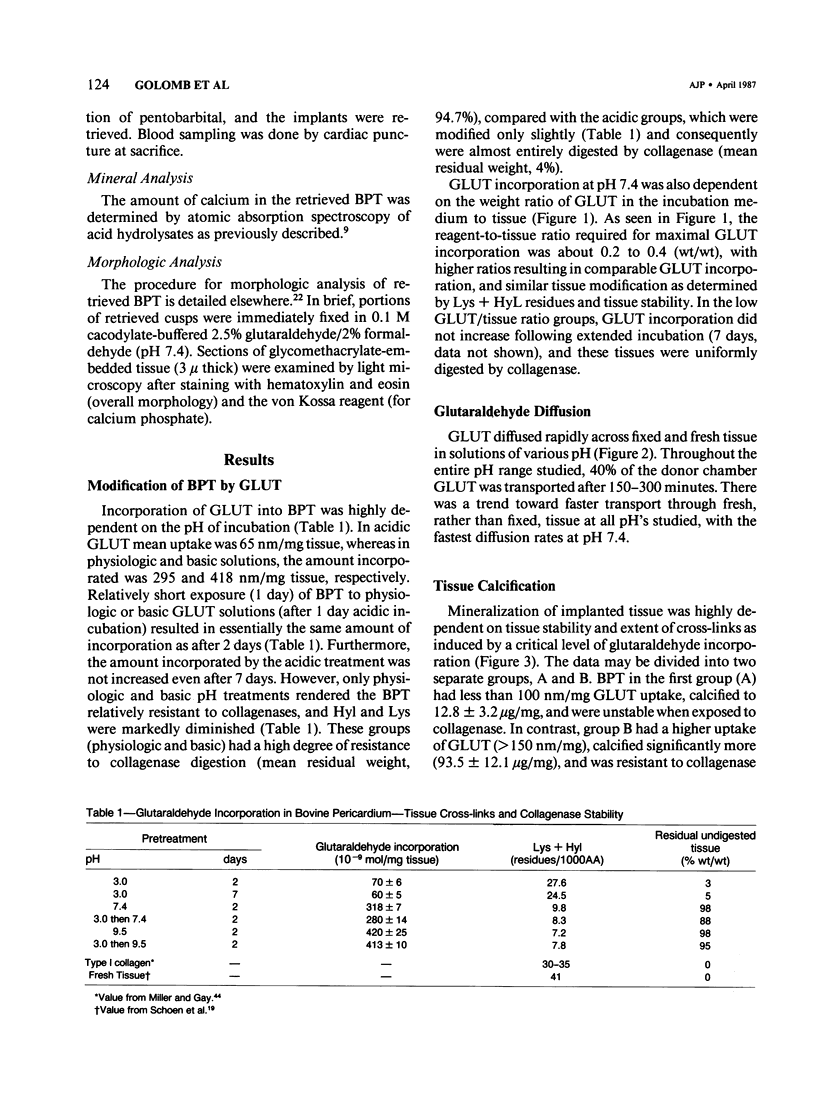
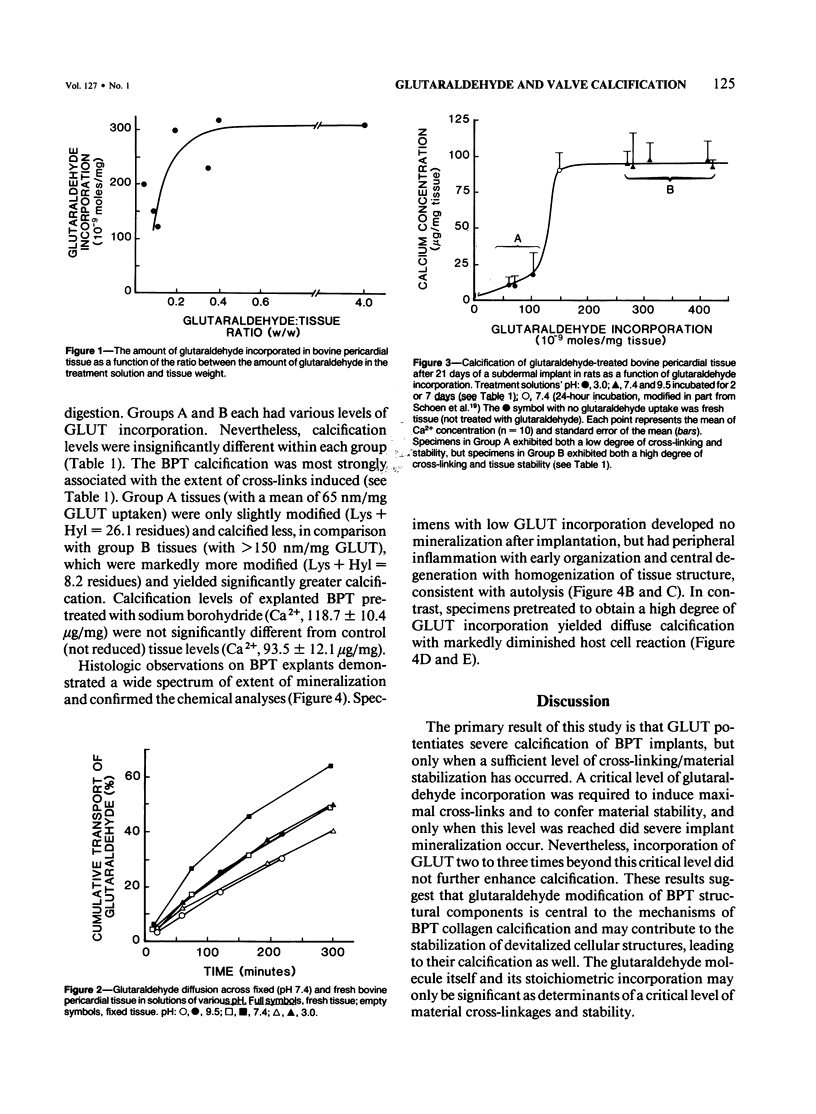
Calcification is the principal cause of failure of tissue-derived cardiac valve replacements pretreated with glutaraldehyde (GLUT). The objective of this study was to determine the role of GLUT-induced cross-links in bovine pericardial tissue calcification. Various levels of 3H-GLUT incorporation were obtained by varying incubation pH, and protein modification was determined by amino acid analysis and resistance to collagenase digestion. Calcification of cross-linked tissue was studied using subdermal implants in rats. Low GLUT uptake (less than 150 nm/mg) resulted in minimal calcification (Ca2+, 12.8 micrograms/mg) and stability (4% residual weight following digestion) due to a limited crosslinking (lysine + hydroxylysine = 26.1 residues/1000 amino acids [AA]). In contrast, higher GLUT uptake induced more cross-links (Lys + Hyl = 8.2 residues/1000 AA) and consequent higher stability (95% residual wt); such tissues calcified severely (Ca2+, 93.5 micrograms/mg). Incorporation of GLUT two to three times beyond a critical level did not further enhance calcification. It is concluded that the amount of GLUT incorporated controls the extent of cross-links, which in turn directly determines tissue stability and calcification.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Mineralization by matrix vesicles. Scan Electron Microsc. 1984;(Pt 2):953–964. [PubMed] [Google Scholar]
- Bowes J. H., Cater C. W. The interaction of aldehydes with collagen. Biochim Biophys Acta. 1968 Oct 21;168(2):341–352. doi: 10.1016/0005-2795(68)90156-6. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Bowling M. C., Grimley P. M. Glutaraldehyde fixation in routine histopathology. Arch Pathol. 1968 Jan;85(1):18–30. [PubMed] [Google Scholar]
- Cheung D. T., Nimni M. E. Mechanism of crosslinking of proteins by glutaraldehyde I: reaction with model compounds. Connect Tissue Res. 1982;10(2):187–199. doi: 10.3109/03008208209034418. [DOI] [PubMed] [Google Scholar]
- Cheung D. T., Nimni M. E. Mechanism of crosslinking of proteins by glutaraldehyde II. Reaction with monomeric and polymeric collagen. Connect Tissue Res. 1982;10(2):201–216. doi: 10.3109/03008208209034419. [DOI] [PubMed] [Google Scholar]
- Cheung D. T., Perelman N., Ko E. C., Nimni M. E. Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connect Tissue Res. 1985;13(2):109–115. doi: 10.3109/03008208509152389. [DOI] [PubMed] [Google Scholar]
- Cohn L. H., Allred E. N., Cohn L. A., Austin J. C., Sabik J., DiSesa V. J., Shemin R. J., Collins J. J., Jr Early and late risk of mitral valve replacement. A 12 year concomitant comparison of the porcine bioprosthetic and prosthetic disc mitral valves. J Thorac Cardiovasc Surg. 1985 Dec;90(6):872–881. [PubMed] [Google Scholar]
- Ferrans V. J., Boyce S. W., Billingham M. E., Jones M., Ishihara T., Roberts W. C. Calcific deposits in porcine bioprostheses: structure and pathogenesis. Am J Cardiol. 1980 Nov;46(5):721–734. doi: 10.1016/0002-9149(80)90421-x. [DOI] [PubMed] [Google Scholar]
- Friedman M., Golomb G. New sustained release dosage form of chlorhexidine for dental use. I. Development and kinetics of release. J Periodontal Res. 1982 May;17(3):323–328. doi: 10.1111/j.1600-0765.1982.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Gallo I., Nistal F., Revuelta J. M., Garcia-Satue E., Artiñano E., Duran C. G. Incidence of primary tissue valve failure with the Ionescu-Shiley pericardial valve. Preliminary results. J Thorac Cardiovasc Surg. 1985 Aug;90(2):278–280. [PubMed] [Google Scholar]
- Gavilanes J. G., González de Buitrago G., Lizarbe M. A., Municio A. M., Olmo N. Stabilization of pericardial tissue by glutaraldehyde. Connect Tissue Res. 1984;13(1):37–44. doi: 10.3109/03008208409152141. [DOI] [PubMed] [Google Scholar]
- Hagler H. K., Lopez L. E., Murphy M. E., Greico C. A., Buja L. M. Quantitative x-ray microanalysis of mitochondrial calcification in damaged myocardium. Lab Invest. 1981 Sep;45(3):241–247. [PubMed] [Google Scholar]
- Harasaki H., McMahon J., Richards T., Goldcamp J., Kiraly R., Nose Y. Calcification in cardiovascular implants: degraded cell related phenomena. Trans Am Soc Artif Intern Organs. 1985;31:489–494. [PubMed] [Google Scholar]
- Hardy P. M., Nicholls A. C., Rydon H. N. The nature of the cross-linking of proteins by glutaraldehyde. Part I. Interaction of glutaraldehyde with the amino-groups of 6-aminohexanoic acid and of alpha-N-acetyl-lysine. J Chem Soc Perkin 1. 1976;(9):958–962. doi: 10.1039/p19760000958. [DOI] [PubMed] [Google Scholar]
- Holloway C. E., Dean F. H. Letter: 13-C-NMR study of aqueous glutaraldehyde equilibria. J Pharm Sci. 1975 Jun;64(6):1078–1079. doi: 10.1002/jps.2600640657. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Callen C. R., McCabe M. The reactions between glutaraldehyde and various proteins. An investigation of their kinetics. Histochem J. 1970 Mar;2(2):137–150. doi: 10.1007/BF01003541. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Theoretical and practical aspects of glutaraldehyde fixation. Histochem J. 1972 Jul;4(4):267–303. doi: 10.1007/BF01005005. [DOI] [PubMed] [Google Scholar]
- Ionescu M. I., Smith D. R., Hasan S. S., Chidambaram M., Tandon A. P. Clinical durability of the pericardial xenograft valve: ten years experience with mitral replacement. Ann Thorac Surg. 1982 Sep;34(3):265–277. doi: 10.1016/s0003-4975(10)62496-4. [DOI] [PubMed] [Google Scholar]
- Korn A. H., Feairheller S. H., Filachione E. M. Glutaraldehyde: nature of the reagent. J Mol Biol. 1972 Apr 14;65(3):525–529. doi: 10.1016/0022-2836(72)90206-9. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Hawley M. A., Schoen F. J., Lund S. A., Liu P. Y. Inhibition by diphosphonate compounds of calcification of porcine bioprosthetic heart valve cusps implanted subcutaneously in rats. Circulation. 1985 Feb;71(2):349–356. doi: 10.1161/01.cir.71.2.349. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Schoen F. J., Levy J. T., Nelson A. C., Howard S. L., Oshry L. J. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983 Nov;113(2):143–155. [PMC free article] [PubMed] [Google Scholar]
- Levy R. J., Schoen F. J., Sherman F. S., Nichols J., Hawley M. A., Lund S. A. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am J Pathol. 1986 Jan;122(1):71–82. [PMC free article] [PubMed] [Google Scholar]
- Levy R. J., Wolfrum J., Schoen F. J., Hawley M. A., Lund S. A., Langer R. Inhibition of calcification of bioprosthetic heart valves by local controlled-release diphosphonate. Science. 1985 Apr 12;228(4696):190–192. doi: 10.1126/science.3919445. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Zenker J. A., Lian J. B. Vitamin K-dependent calcium binding proteins in aortic valve calcification. J Clin Invest. 1980 Feb;65(2):563–566. doi: 10.1172/JCI109700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magilligan D. J., Jr, Lewis J. W., Jr, Tilley B., Peterson E. The porcine bioprosthetic valve. Twelve years later. J Thorac Cardiovasc Surg. 1985 Apr;89(4):499–507. [PubMed] [Google Scholar]
- Milano A., Bortolotti U., Talenti E., Valfrè C., Arbustini E., Valente M., Mazzucco A., Gallucci V., Thiene G. Calcific degeneration as the main cause of porcine bioprosthetic valve failure. Am J Cardiol. 1984 Apr 1;53(8):1066–1070. doi: 10.1016/0002-9149(84)90638-6. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. Collagen: an overview. Methods Enzymol. 1982;82(Pt A):3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Reul G. J., Jr, Cooley D. A., Duncan J. M., Frazier O. H., Hallman G. L., Livesay J. J., Ott D. A., Walker W. E. Valve failure with the Ionescu-Shiley bovine pericardial bioprosthesis: analysis of 2680 patients. J Vasc Surg. 1985 Jan;2(1):192–204. [PubMed] [Google Scholar]
- Sanders S. P., Levy R. J., Freed M. D., Norwood W. I., Castaneda A. R. Use of Hancock porcine xenografts in children and adolescents. Am J Cardiol. 1980 Sep;46(3):429–438. doi: 10.1016/0002-9149(80)90012-0. [DOI] [PubMed] [Google Scholar]
- Schoen F. J., Levy R. J. Bioprosthetic heart valve failure: pathology and pathogenesis. Cardiol Clin. 1984 Nov;2(4):717–739. [PubMed] [Google Scholar]
- Schoen F. J., Levy R. J., Nelson A. C., Bernhard W. F., Nashef A., Hawley M. Onset and progression of experimental bioprosthetic heart valve calcification. Lab Invest. 1985 May;52(5):523–532. [PubMed] [Google Scholar]
- Schoen F. J., Tsao J. W., Levy R. J. Calcification of bovine pericardium used in cardiac valve bioprostheses. Implications for the mechanisms of bioprosthetic tissue mineralization. Am J Pathol. 1986 Apr;123(1):134–145. [PMC free article] [PubMed] [Google Scholar]
- Silver M., Hudson R. E., Trimble A. S. Morphologic observations on heart valve prostheses made of fascia lata. J Thorac Cardiovasc Surg. 1975 Aug;70(2):360–366. [PubMed] [Google Scholar]
- Valente M., Bortolotti U., Thiene G. Ultrastructural substrates of dystrophic calcification in porcine bioprosthetic valve failure. Am J Pathol. 1985 Apr;119(1):12–21. [PMC free article] [PubMed] [Google Scholar]
- Woodroof E. A. Use of glutaraldehyde and formaldehyde to process tissue heart valves. J Bioeng. 1978 Apr;2(1-2):1–9. [PubMed] [Google Scholar]



