Abstract
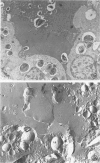
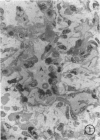
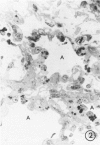
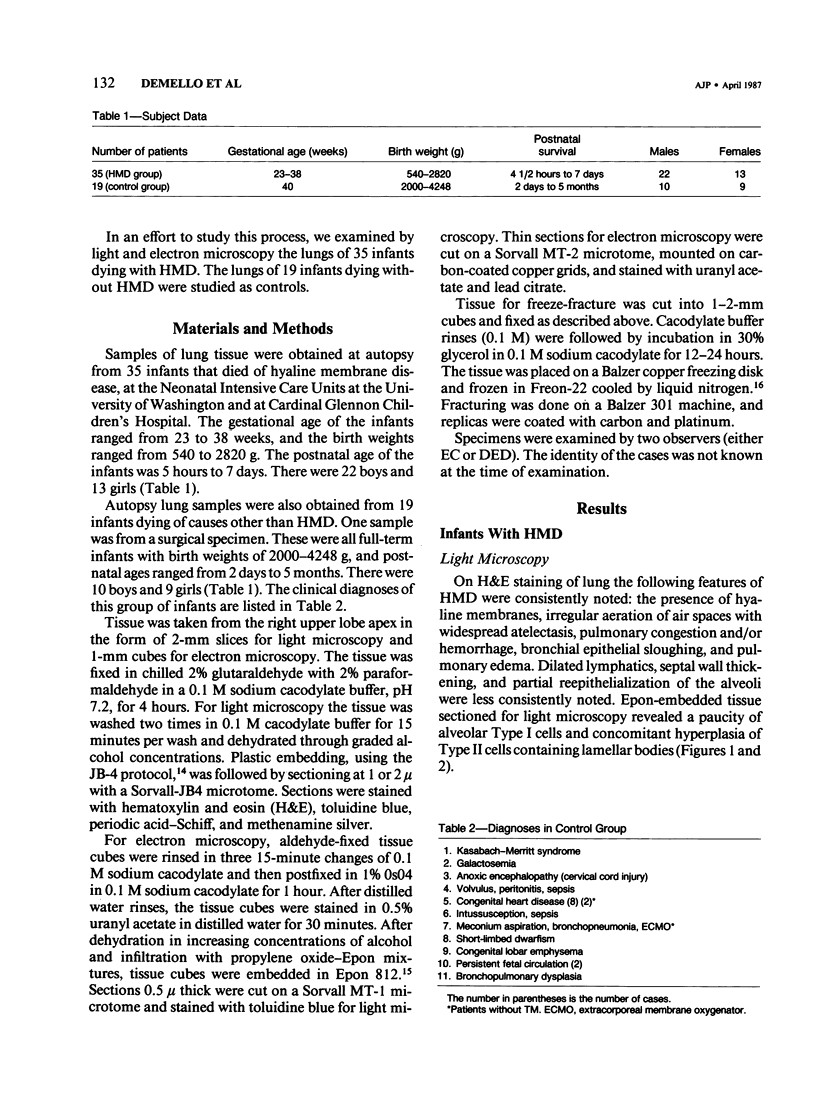
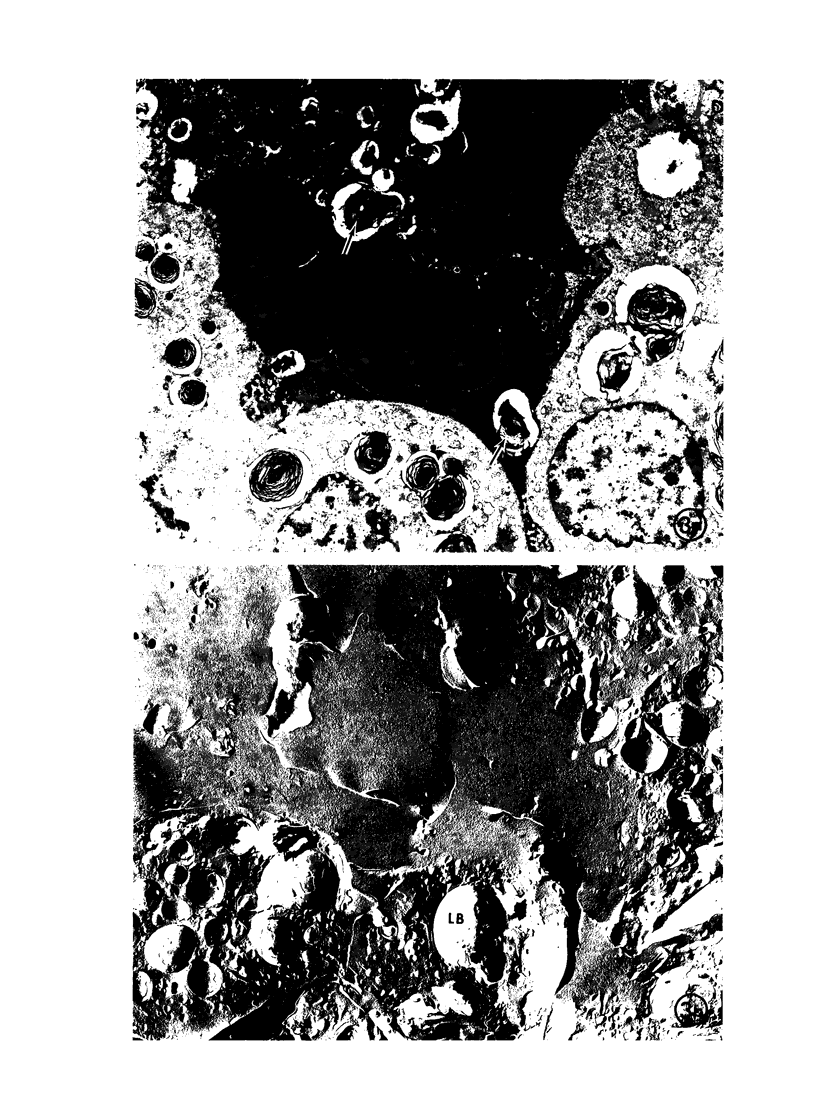
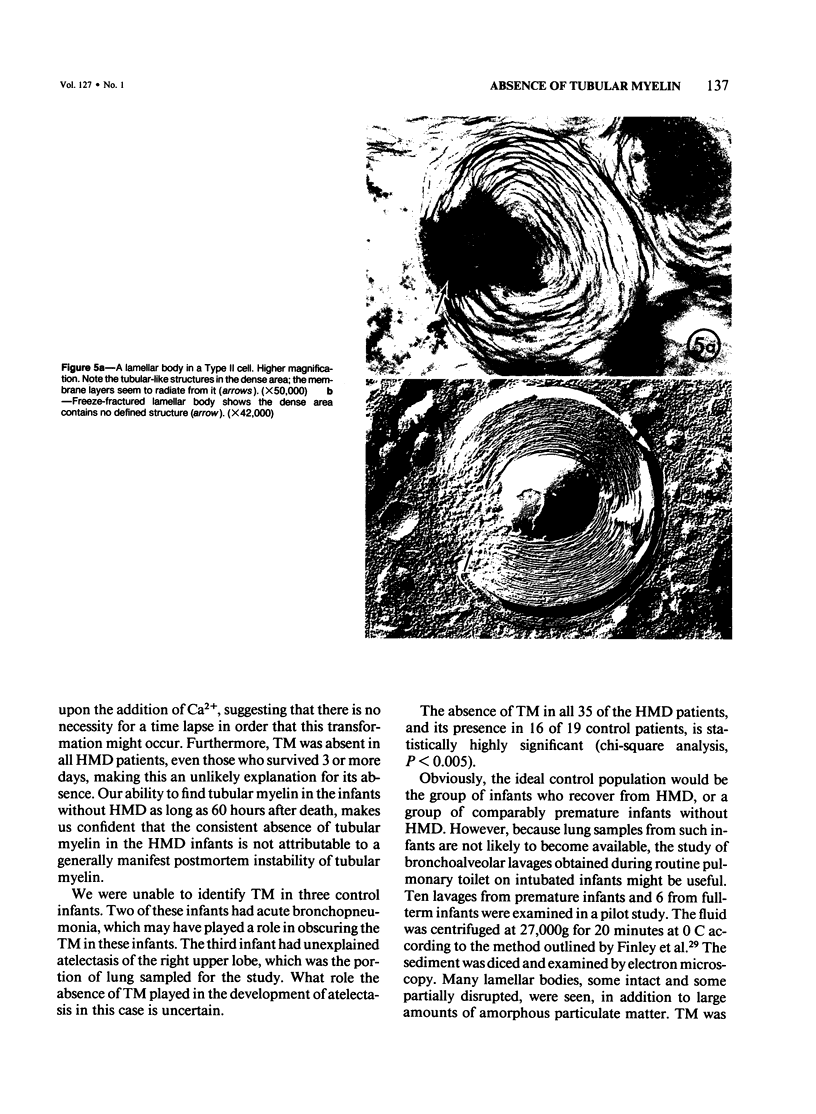
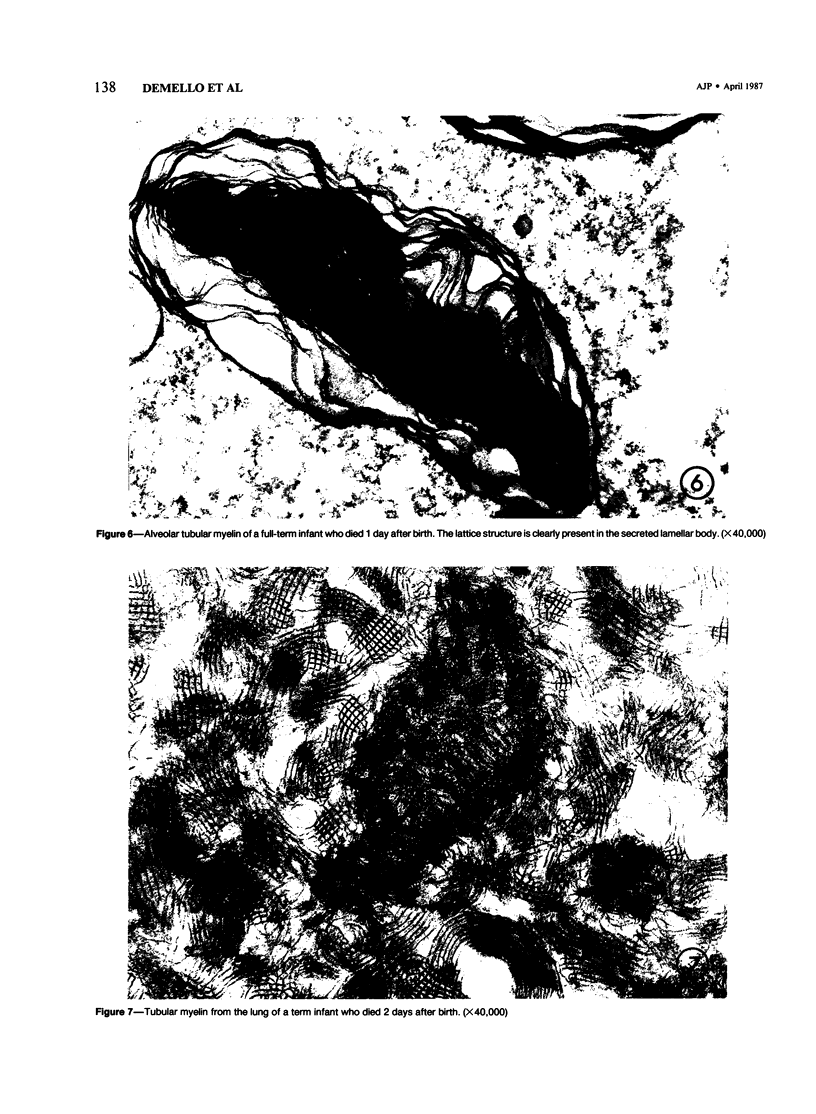
Immaturity of the pulmonary surface active material synthesizing system with deficiency of surface active material in the premature lung is an accepted cause of hyaline membrane disease. Lamellar bodies, the intracellular form of surface active material, are produced and secreted from Type II pneumocytes and converted to tubular myelin in the alveolar lumen. Tubular myelin, in turn, gives rise to the surface monolayer, which has the greatest surface active property. Thus, lung sections were studied by light and electron microscopy from 35 infants who died of histologically confirmed hyaline membrane disease and 19 infants who died of other causes. Tubular myelin was not identified ultrastructurally in lungs of infants who died of hyaline membrane disease, despite the presence of abundant lamellar bodies. In contrast, 16 of 19 infants dying of other causes had easily identifiable tubular myelin in addition to lamellar bodies. The absence of tubular myelin in the hyaline membrane disease patients suggests an abnormality in the conversion of lamellar bodies to tubular myelin. The authors speculate that this abnormal lamellar body turnover may be important in the pathogenesis of hyaline membrane disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Anderson W. R., Strickland M. B., Tsai S. H., Haglin J. J. Light microscopic and ultrastructural study of the adverse effects of oxygen therapy on the neonate lung. Am J Pathol. 1973 Nov;73(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R., Roos B. Postmortem fixation of human lungs for electron microscopy. Am Rev Respir Dis. 1975 Mar;111(3):247–256. doi: 10.1164/arrd.1975.111.3.247. [DOI] [PubMed] [Google Scholar]
- Balis J. U., Delivoria M., Conen P. E. Maturation of postnatal human lung and the idiopathic respiratory distress syndrome. Lab Invest. 1966 Mar;15(3):530–546. [PubMed] [Google Scholar]
- CLEMENTS J. A. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957 May;95(1):170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Abnormally large lamellar bodies in type II pneumocytes in Chediak-Higashi syndrome in beige mice. Lab Invest. 1976 Feb;34(2):166–173. [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D. Linear arrays of intramembranous particles in pulmonary tubular myelin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6225–6229. doi: 10.1073/pnas.75.12.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Smuckler E. A. A rapid method for processing liver biopsy specimens for 2 micron sectioning. Arch Pathol Lab Med. 1976 Sep;100(9):457–462. [PubMed] [Google Scholar]
- Farrell P. M., Avery M. E. Hyaline membrane disease. Am Rev Respir Dis. 1975 May;111(5):657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- Finley T. N., Pratt S. A., Ladman A. J., Brewer L., McKay M. B. Morphological and lipid analysis of the alveolar lining material in dog lung. J Lipid Res. 1968 May;9(3):357–365. [PubMed] [Google Scholar]
- Gil J. Histological preservation and ultrastructure of alveolar surfactant. Annu Rev Physiol. 1985;47:753–763. doi: 10.1146/annurev.ph.47.030185.003541. [DOI] [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groniowski J. A. Fine structural basis of pulmonary surfactant. Int Rev Exp Pathol. 1983;25:183–238. [PubMed] [Google Scholar]
- Groniowski J. Presumable role of surface glycoprotein of alveolar pneumonocytes in transformation of lamellar bodies into tubular myelin. Acta Med Pol. 1979;20(4):393–396. [PubMed] [Google Scholar]
- Groniowski J., Walski M. Further studies on the tubular myelin structure of extracellular alveolar lining layer. Acta Med Pol. 1979;20(1):15–16. [PubMed] [Google Scholar]
- Hallman M., Feldman B. H., Kirkpatrick E., Gluck L. Absence of phosphatidylglycerol (PG) in respiratory distress syndrome in the newborn. Study of the minor surfactant phospholipids in newborns. Pediatr Res. 1977 Jun;11(6):714–720. doi: 10.1203/00006450-197706000-00003. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B., Talley F. A. Multilamelled structures from the lungs of patients with pulmonary alveolar proteinosis. Lab Invest. 1984 Jun;50(6):711–725. [PubMed] [Google Scholar]
- Kikkawa Y. Morphology of alveolar lining layer. Anat Rec. 1970 Aug;167(4):389–400. doi: 10.1002/ar.1091670403. [DOI] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns J. M. "Hyaline membrane disease" in newborn infants. Macroscopic, radiographic, and light and electron microscopic studies. Hum Pathol. 1970 Jun;1(2):175–204. doi: 10.1016/s0046-8177(70)80033-8. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function and origin of the alveolar lining layer. Nature. 1955 Jun 25;175(4469):1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- Paul G. W., Hassett R. J., Reiss O. K. Formation of lung surfactant films from intact lamellar bodies. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3617–3620. doi: 10.1073/pnas.74.8.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. L., Hassett R. J., Vatter A. E. Isolation of lung lamellar bodies and their conversion to tubular myelin figures in vitro. Anat Rec. 1980 Nov;198(3):485–501. doi: 10.1002/ar.1091980310. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Vatter A. E. A mode of formation of tubular myelin from lamellar bodies in the lung. J Cell Biol. 1977 Sep;74(3):1027–1031. doi: 10.1083/jcb.74.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley S. A., Kovacevic M., Paciga J. E., Balis J. U. Sequential changes of surfactant phosphatidylcholine in hyaline-membrane disease of the newborn. N Engl J Med. 1979 Jan 18;300(3):112–116. doi: 10.1056/NEJM197901183000303. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Gil J. Electron microscopic demonstration of an extracellular duplex lining layer of alveoli. Respir Physiol. 1968 Jan;4(1):42–57. doi: 10.1016/0034-5687(68)90006-6. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Töndury G. A stereologic electron microscope study of "tubular myelin figures" in alveolar fluids of rat lungs. Z Zellforsch Mikrosk Anat. 1966;69:418–427. doi: 10.1007/BF00406293. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Freeze-fracture studies of tubular myelin and lamellar bodies in fetal and adult rat lungs. J Ultrastruct Res. 1978 Sep;64(3):352–361. doi: 10.1016/s0022-5320(78)90043-6. [DOI] [PubMed] [Google Scholar]
- Williams M. C., Mason R. J. Development of the type II cell in the fetal rat lung. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):37–47. doi: 10.1164/arrd.1977.115.S.37. [DOI] [PubMed] [Google Scholar]