Abstract
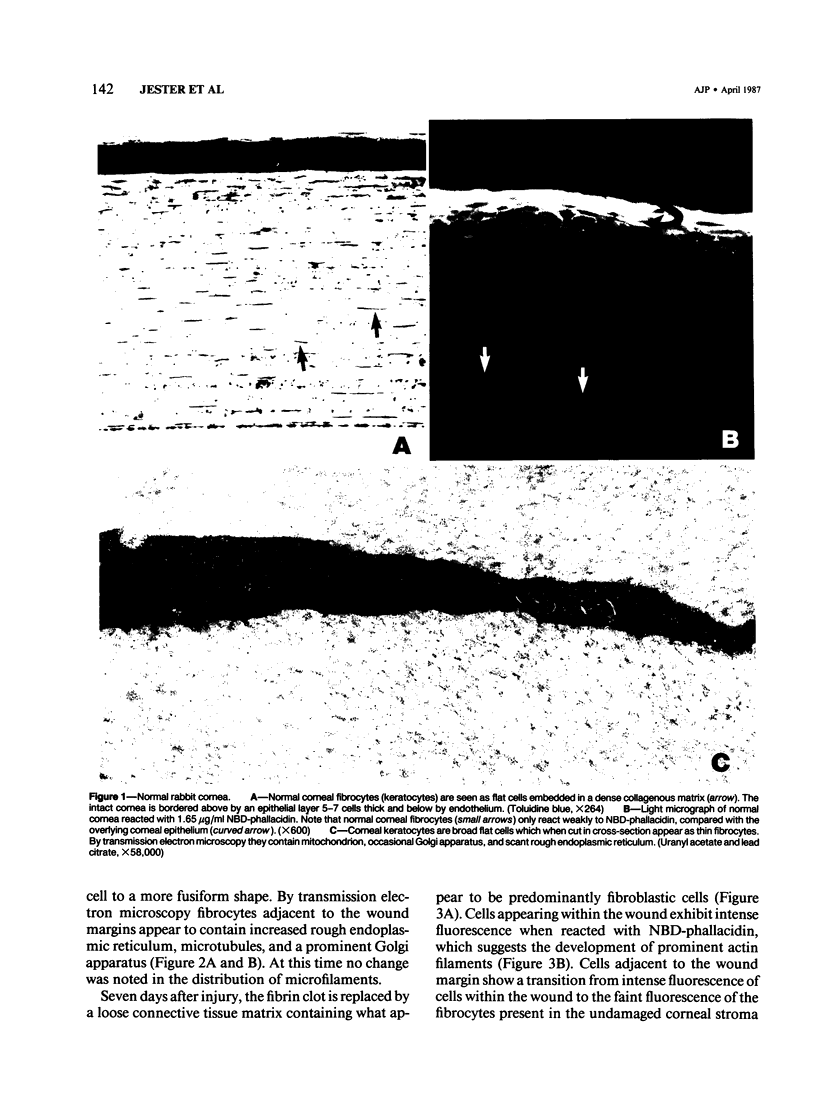
The characteristics and derivation of corneal wound healing fibroblasts (myofibroblasts) were evaluated by studying the temporal changes in the cellular actin distribution of corneal fibrocytes following full thickness 3-mm diameter central corneal wounds in the rabbit. Under certain conditions these wounds heal without neovascularization, allowing for the detailed analysis of invading fibroblasts with minimal contamination by other cell types. The authors employed transmission electron microscopy to localize microfilaments, fluorescent microscopy using NBD-phallacidin, a mushroom toxin which binds specifically to f-actin and oligomeres of g-actin, to localize actin filaments, and isoelectric focusing gels to characterize actin isotypes. During the early stages of wound healing (1-7 days) there is a gradual change in the corneal fibrocytes adjacent to the wound margin characterized by the development of extensive rough endoplasmic reticulum, microtubules, a prominent Golgi apparatus, and a cortical microfilament network. This is in contrast to the normal fibrocyte, which, for the most part, lacks these structures. The development of microfilaments correlated with increased NBD-phallacidin fluorescence of fibrocytes adjacent to the wound as compared with fibrocytes farther removed from the site of injury. Fibroblasts appearing within the wound from 7 days to 2 months after injury had ultrastructural characteristics similar to those of myofibroblasts, including parallel arrays of microfilaments, stress fibers and cell-cell, cell-matrix attachments. Furthermore, these cells stained intensely with NBD-phallacidin, supporting the ultrastructural findings. At 1 month after injury, cells contained within the wound possessed predominantly nonmuscle isoactins (gamma) as seen by silver staining of isoelectric focusing gels, but little or no (smooth muscle) isoactins could be detected. Moreover, no significant differences could be detected between electrophoretic profiles obtained from wounded versus normal corneas. These morphologic and biochemical data suggest that the corneal fibrocyte may develop into a fibroblastlike cell similar to the myofibroblast, and is characterized by a marked increase in filamentous actin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell G. R., Ryan G. B. Origin of myofibroblasts in the avascular capsule around free-floating intraperitoneal blood clots. Pathology. 1983 Jul;15(3):253–264. doi: 10.3109/00313028309083503. [DOI] [PubMed] [Google Scholar]
- Cintron C., Schneider H., Kublin C. Corneal scar formation. Exp Eye Res. 1973 Nov 11;17(3):251–259. doi: 10.1016/0014-4835(73)90176-0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Hüttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978 Mar;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Heimark R. L., Schwartz S. M. Organization of actin cytoskeleton during early endothelial regeneration in vitro. J Cell Sci. 1984 Mar;66:39–50. doi: 10.1242/jcs.66.1.39. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D., Wong A. J. Contractile proteins in endothelial cells. Ann N Y Acad Sci. 1982;401:50–60. doi: 10.1111/j.1749-6632.1982.tb25706.x. [DOI] [PubMed] [Google Scholar]
- Kuroda M. Change of actin isomers during differentiation of smooth muscle. Biochim Biophys Acta. 1985 Dec 13;843(3):208–213. doi: 10.1016/0304-4165(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Low R. B., Chaponnier C., Gabbiani G. Organization of actin in epithelial cells during regenerative and neoplastic conditions. Correlation of morphologic, immunofluorescent, and biochemical findings. Lab Invest. 1981 Apr;44(4):359–367. [PubMed] [Google Scholar]
- Matsuda H., Smelser G. K. Electron microscopy of corneal wound healing. Exp Eye Res. 1973 Sep;16(6):427–442. doi: 10.1016/0014-4835(73)90100-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irle C., Statkov P. R., Majno G. Myofibroblasts in an avascular fibrous tissue. Lab Invest. 1973 Aug;29(2):197–206. [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irlé C., Montandon D., Statkov P. R., Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974 Jan;5(1):55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Thelmo W. L. The myofibroblast: biologic, pathologic, and theoretical considerations. Pathol Annu. 1980;15(Pt 1):443–470. [PubMed] [Google Scholar]
- Sottiurai V. S., Yao J. S., Flinn W. R., Batson R. C. Intimal hyperplasia and neointima: An ultrastructural analysis of thrombosed grafts in humans. Surgery. 1983 Jun;93(6):809–817. [PubMed] [Google Scholar]
- Squier C. A., Leranth C. S., Ghoneim S., Kremenak C. R. Electron microscopic immunochemical localization of actin in fibroblasts in healing skin and palate wounds of beagle dog. Histochemistry. 1983;78(4):513–522. doi: 10.1007/BF00496203. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]