Abstract
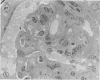
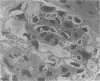
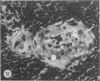
The type of dietary fat dramatically affects the onset of autoimmune disease in lupus-prone female New Zealand Black/New Zealand White F1 (B/W) mice. Disease development was strikingly slowed in mice fed a diet containing quantities of omega-3 fatty acids (fish oil, FO). By 10 months of age, 94% of the FO mice were still living, whereas all the mice fed a saturated fat diet (lard,L) were dead. Those mice fed a corn oil (CO) diet were intermediate with 35% alive at the 10-month time evaluation. Long after the L and CO groups had succumbed to glomerulonephritis, the FO group had negligible proteinuria. Both B and T cell function, particularly antibody production and resultant circulating immune complex (CIC) levels, were modified by the type of dietary fat. FO mice exhibited lower levels of anti-ds-DNA and lower levels of CICs than L or CO mice. B/W antibody response to a T-independent antigen (DNP-dextran) was enhanced at 8 months of age in FO mice, whereas it was suppressed in L mice. T-dependent (sheep red blood cell) responses at that time period were reduced in all the diet groups, a reflection of the reduced numbers of accessory T cells as determined by FACS analysis. The natural killer (NK) response to YAC-1 cells decreased in the L group from 5 to 9 months of age but remained unchanged in the CO and FO groups. Severe glomerulonephritis was the most common histopathologic finding in the L and CO groups. Arteritis was found in the spleens of nearly all the L and CO mice. Arteritis of the heart, colon and intestine, stomach, kidney, and liver were also seen principally in the L mice. In contrast, most FO mice had minimal to mild glomerulonephritis and no or minimal arteritis in the spleen. It is likely omega-3 fatty acids of fish oil reduce immune-complex-induced glomerulonephritis through production of prostaglandin metabolites with attenuated activity and/or through altering cell membrane structure and fluidity, which may, in turn, affect the responsiveness of immune cells.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., de Groot E. R., Feltkamp T. E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975 Jun 30;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- Alexander N. J., Clarkson T. B., Fulgham D. L. Circulating immune complexes in cynomolgus macaques. Lab Anim Sci. 1985 Oct;35(5):465–468. [PubMed] [Google Scholar]
- Anderson C. L., Stillman W. S. Raji cell assay for immune complexes. Evidence for detection of Raji-directed immunoglobulin G antibody in sera from patients with systemic lupus erythematosus. J Clin Invest. 1980 Aug;66(2):353–360. doi: 10.1172/JCI109863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel M., Charriaut C., Frade R. Use of cell differentiation effectors to select a human B lymphoblastoid cell line enriched in C3b receptors. J Immunol Methods. 1981;46(2):187–195. doi: 10.1016/0022-1759(81)90135-6. [DOI] [PubMed] [Google Scholar]
- Barkas T. A simple, rapid and sensitive assay for immune complexes using a Staphylococcus aureus immunoadsorbent. J Clin Lab Immunol. 1981 Jan;5(1):59–65. [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Chen I. S., Subramaniam S., Cassidy M. M., Sheppard A. J., Vahouny G. V. Intestinal absorption and lipoprotein transport of (omega-3) eicosapentaenoic acid. J Nutr. 1985 Feb;115(2):219–225. doi: 10.1093/jn/115.2.219. [DOI] [PubMed] [Google Scholar]
- Evans M. M., Williamson W. G., Irvine W. J. The appearance of immunological competence at an early age in New Zealand black mice. Clin Exp Immunol. 1968 Jun;3(5):375–383. [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Smith J., Good R. A. Dietary influence on breeding behavior, hemolytic anemia, and longevity in NZB mice. Proc Soc Exp Biol Med. 1972 Apr;139(4):1189–1196. doi: 10.3181/00379727-139-36327. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pickett W. C., Goetzl E. J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983 Nov 30;117(1):282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd E. R., Johnston J. M., Okita J. R., MacDonald P. C., Ziff M., Gilliam J. W. Prevention of glomerulonephritis and prolonged survival in New Zealand Black/New Zealand White F1 hybrid mice fed an essential fatty acid-deficient diet. J Clin Invest. 1981 Feb;67(2):476–485. doi: 10.1172/JCI110056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth D. R., Harris W. S., Connor W. E. Inhibition of low density lipoprotein synthesis by dietary omega-3 fatty acids in humans. Arteriosclerosis. 1984 May-Jun;4(3):270–275. doi: 10.1161/01.atv.4.3.270. [DOI] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Fischer D. G., Copeland C. S., Jensen P. J. Regulation of human natural killing. I. The role of monocytes, interferon, and prostaglandins. J Immunol. 1981 Nov;127(5):2007–2013. [PubMed] [Google Scholar]
- Kumar V., Krasny S., Beutner E. H. Specificity of the Crithidia luciliae method for detecting anti-DNA antibodies. Effect of absorption for lipoproteins. Immunol Invest. 1985 Jun;14(3):199–210. doi: 10.3109/08820138509076144. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Ibrahim A. B., Shirai T., Ohta K., Nagasawa R., Yoshida H., Estes J., Gardner M. Dietary fat affects immune response, production of antiviral factors, and immune complex disease in NZB/NZW mice. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1974–1978. doi: 10.1073/pnas.79.6.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar V., Brown E., Leiserson W. M., Chused T. M. Expression of Lyt-1 by a subset of B lymphocytes. J Immunol. 1982 Aug;129(2):532–538. [PubMed] [Google Scholar]
- Marshall L. A., Johnston P. V. The effect of dietary alpha-linolenic acid in the rat on fatty acid profiles of immunocompetent cell populations. Lipids. 1983 Oct;18(10):737–742. doi: 10.1007/BF02534542. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Gershwin M. E. The pathogenesis of autoimmunity in New Zealand mice. Semin Arthritis Rheum. 1980 Nov;10(2):111–147. doi: 10.1016/0049-0172(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Morrow W. J., Ohashi Y., Hall J., Pribnow J., Hirose S., Shirai T., Levy J. A. Dietary fat and immune function. I. Antibody responses, lymphocyte and accessory cell function in (NZB x NZW)F1 mice. J Immunol. 1985 Dec;135(6):3857–3863. [PubMed] [Google Scholar]
- Nagy L., Lee T. H., Goetzl E. J., Pickett W. C., Kay A. B. Complement receptor enhancement and chemotaxis of human neutrophils and eosinophils by leukotrienes and other lipoxygenase products. Clin Exp Immunol. 1982 Mar;47(3):541–547. [PMC free article] [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C., Hudig D., Newton R. S., Mendelsohn J. Effect of unsaturated fatty acids on human lymphocytes: disparate influences of oleic and linolenic acids on natural cytotoxicity. Clin Immunol Immunopathol. 1981 Sep;20(3):389–401. doi: 10.1016/0090-1229(81)90149-5. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Roozemond R. C., Bonavida B. Effect of altered membrane fluidity on NK cell-mediated cytotoxicity. I. Selective inhibition of the recognition or post recognition events in the cytolytic pathway of NK cells. J Immunol. 1985 Apr;134(4):2209–2214. [PubMed] [Google Scholar]
- Slack J. H., Hang L., Barkley J., Fulton R. J., D'Hoostelaere L., Robinson A., Dixon F. J. Isotypes of spontaneous and mitogen-induced autoantibodies in SLE-prone mice. J Immunol. 1984 Mar;132(3):1271–1275. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Eisenberg R. A., Bourdon M., Crowell J. S., Jr, Dixon F. J. Distribution of lymphocytes identified by surface markers in murine strains with systemic lupus erythematosus-like syndromes. J Exp Med. 1979 Feb 1;149(2):516–534. doi: 10.1084/jem.149.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron F., Jacob L., Bach J. F. Binding of a murine monoclonal anti-DNA antibody to Raji cells. Implications for the interpretation of the Raji cell assay for immune complexes. Eur J Immunol. 1984 Mar;14(3):283–286. doi: 10.1002/eji.1830140316. [DOI] [PubMed] [Google Scholar]
- Wager O., Lindström P., Räsänen J. A., Kekomäki R., Ziola B., Salmi A., Isomäki H., Skrifvars B., Penttinen K. Evaluation of six tests for circulating IgG complexes with special reference to IgM rheumatoid factors: analysis of systemic lupus erythematosus and rheumatoid arthritis series. Clin Exp Immunol. 1981 Oct;46(1):149–160. [PMC free article] [PubMed] [Google Scholar]
- Wene J. D., Connor W. E., DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. J Clin Invest. 1975 Jul;56(1):127–134. doi: 10.1172/JCI108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Zelikovsky G., Bongiovanni A. M., Geller N., Good R. A., Day N. K. Sperm-related antigens, antibodies, and circulating immune complexes in sera of recently vasectomized men. J Clin Invest. 1982 Jul;70(1):33–40. doi: 10.1172/JCI110600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura W., Hattori S., Morrow W. J., Mayes D. C., Levy J. A., Shirai T. Dietary fat and immune function. II. Effects on immune complex nephritis in (NZB x NZW)F1 mice. J Immunol. 1985 Dec;135(6):3864–3868. [PubMed] [Google Scholar]