Abstract
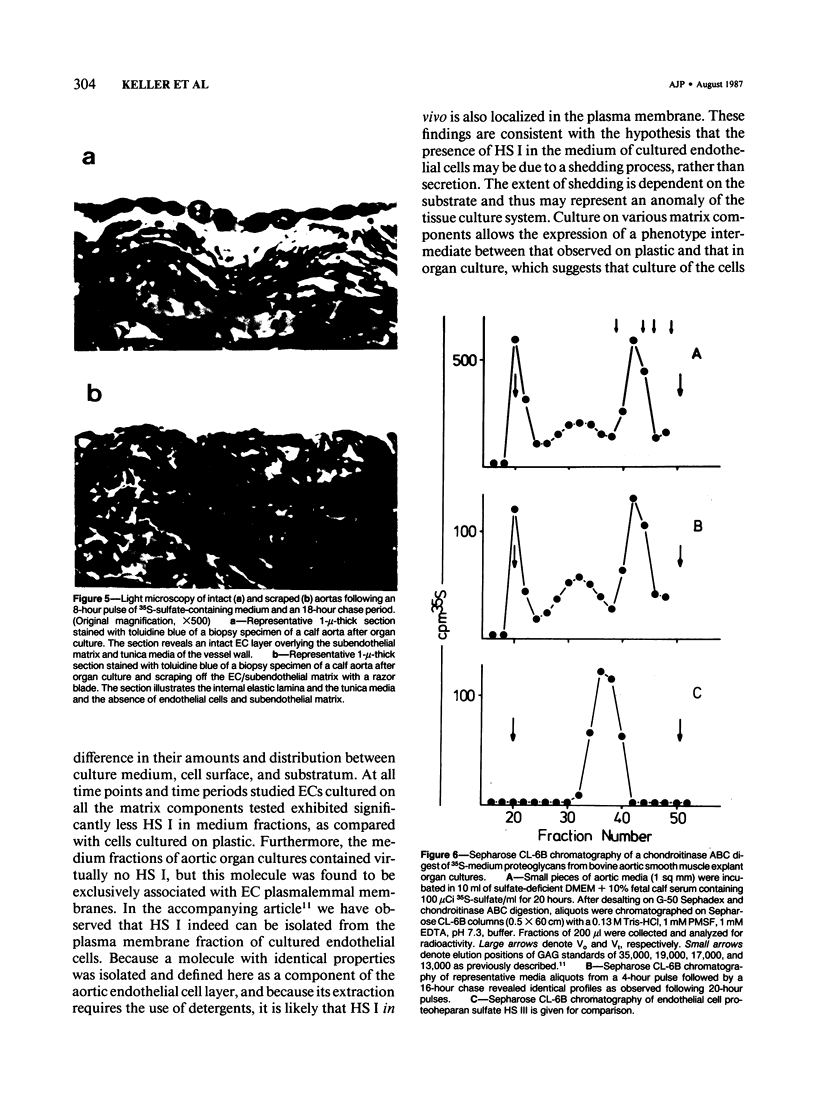
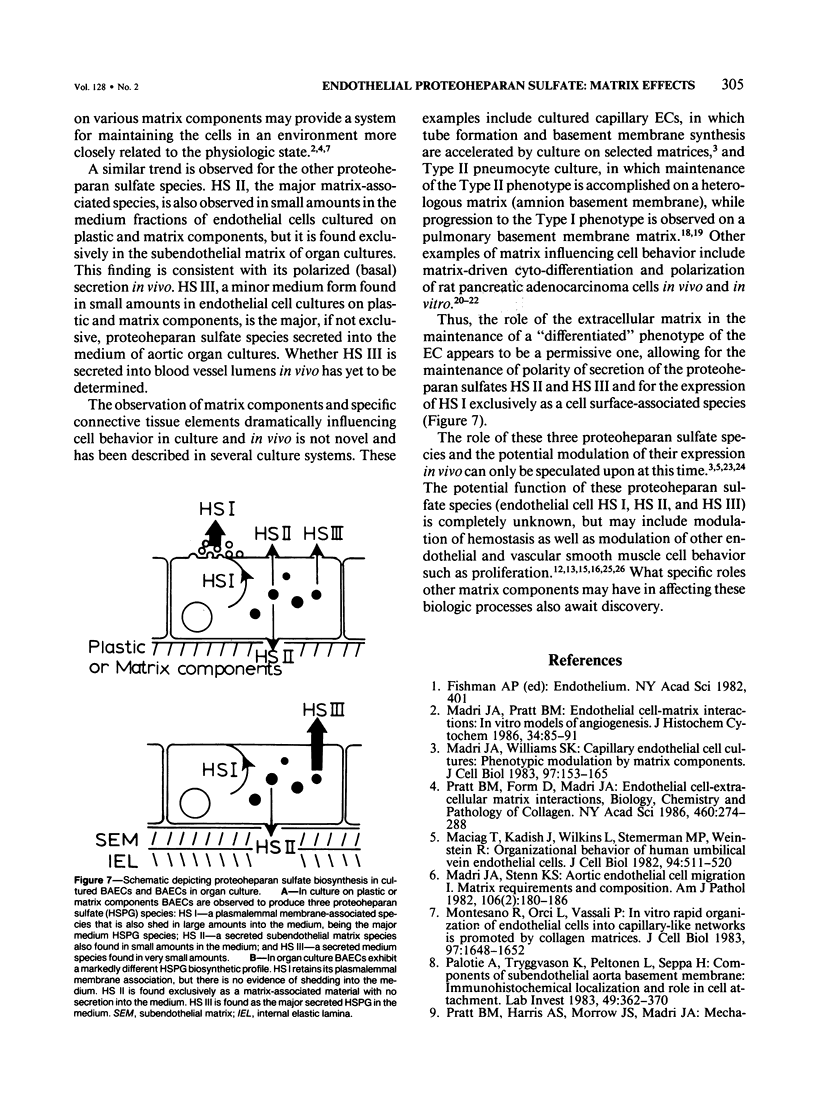
The effects of extracellular matrix components on proteoheparan sulfate biosynthesis was studied for bovine aortic endothelial cells in tissue culture. When the cells were maintained on a variety of different purified components of the extracellular matrix, the cells expressed the same three species of proteoheparan sulfates as the cells cultured on tissue culture plastic (HS I, HS II, and HS III). However, the amounts of the three species recovered from the tissue culture medium were found to be dependent on the substrate on which the cells are grown as well as on other factors. In comparison with plastic, much less HS I was found in the medium of cells maintained on substrates containing diverse matrix molecules, whereas the amounts of HS II and HS III essentially remained the same. In contrast, when bovine aortic organ cultures were analyzed under pulsatile flow, marked differences in the profile of proteoheparan sulfate biosynthesis were observed: HS I was found exclusively associated with the plasma membrane of the endothelial cells; HS II was localized only to the subendothelial matrix; and HS III represented the only proteoheparan sulfate species in the medium. This distribution is consistent with polarized secretion and deposition into the subcellular matrix of HS III and retention of HS I in the plasma membrane in the organ culture situation, a biosynthetic phenotype which can only be approximated at best by maintaining the endothelial cells on a substrate other than plastic. When aortic media (devoid of endothelial cells) was placed in organ culture, no HS III could be detected, which suggested that the vascular endothelial cell is the major cell type responsible for its synthesis in organ culture. Thus, the extracellular matrix, depending upon its composition and organization, may play an important role in stabilizing cell polarity and thereby contribute to maintenance of the differentiated phenotype appropriate for the endothelial cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizkhan R. G., Azizkhan J. C., Zetter B. R., Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980 Oct 1;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonassisi V., Colburn P. Antibodies to the heparan sulfate proteoglycans synthesized by endothelial cell cultures. Biochim Biophys Acta. 1983 Oct 4;760(1):1–12. doi: 10.1016/0304-4165(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Basement membrane as a spatial organizer of polarized epithelia. Exogenous basement membrane reorients pancreatic epithelial tumor cells in vitro. Am J Pathol. 1986 Jan;122(1):129–139. [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Neoplastic disorganization of pancreatic epithelial cell-cell relations. Role of basement membrane. Am J Pathol. 1985 Nov;121(2):248–260. [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J. Endothelial--vascular smooth muscle cell interactions. Rous--Whipple Award Lecture. Am J Pathol. 1981 Dec;105(3):200–206. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Furthmayr H. Isolation and characterization of basement membrane and cell proteoheparan sulphates from HR9 cells. Eur J Biochem. 1986 Dec 15;161(3):707–714. doi: 10.1111/j.1432-1033.1986.tb10497.x. [DOI] [PubMed] [Google Scholar]
- Keller R., Silbert J. E., Furthmayr H., Madri J. A. Aortic endothelial cell proteoheparan sulfate. I. Isolation and characterization of plasmamembrane-associated and extracellular species. Am J Pathol. 1987 Aug;128(2):286–298. [PMC free article] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Ingbar D. H., Madri J. A. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986 Feb;162(2):423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Thulin G., Madri J. A., Barrett C., Warshaw J. B. An acellular human amnionic membrane model for in vitro culture of type II pneumocytes: the role of the basement membrane in cell morphology and function. J Cell Physiol. 1984 Oct;121(1):215–225. doi: 10.1002/jcp.1041210127. [DOI] [PubMed] [Google Scholar]
- Maciag T., Kadish J., Wilkins L., Stemerman M. B., Weinstein R. Organizational behavior of human umbilical vein endothelial cells. J Cell Biol. 1982 Sep;94(3):511–520. doi: 10.1083/jcb.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem. 1986 Jan;34(1):85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Stenn K. S. Aortic endothelial cell migration. I. Matrix requirements and composition. Am J Pathol. 1982 Feb;106(2):180–186. [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983 Nov;97(5 Pt 1):1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotie A., Tryggvason K., Peltonen L., Seppä H. Components of subendothelial aorta basement membrane. Immunohistochemical localization and role in cell attachment. Lab Invest. 1983 Sep;49(3):362–370. [PubMed] [Google Scholar]
- Pratt B. M., Form D., Madri J. A. Endothelial cell-extracellular matrix interactions. Ann N Y Acad Sci. 1985;460:274–288. doi: 10.1111/j.1749-6632.1985.tb51175.x. [DOI] [PubMed] [Google Scholar]
- Pratt B. M., Harris A. S., Morrow J. S., Madri J. A. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984 Dec;117(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L., Kumar S. Importance of a collagen substratum for stimulation of capillary endothelial cell proliferation by tumour angiogenesis factor. Int J Cancer. 1979 Aug;24(2):225–234. doi: 10.1002/ijc.2910240215. [DOI] [PubMed] [Google Scholar]