Abstract
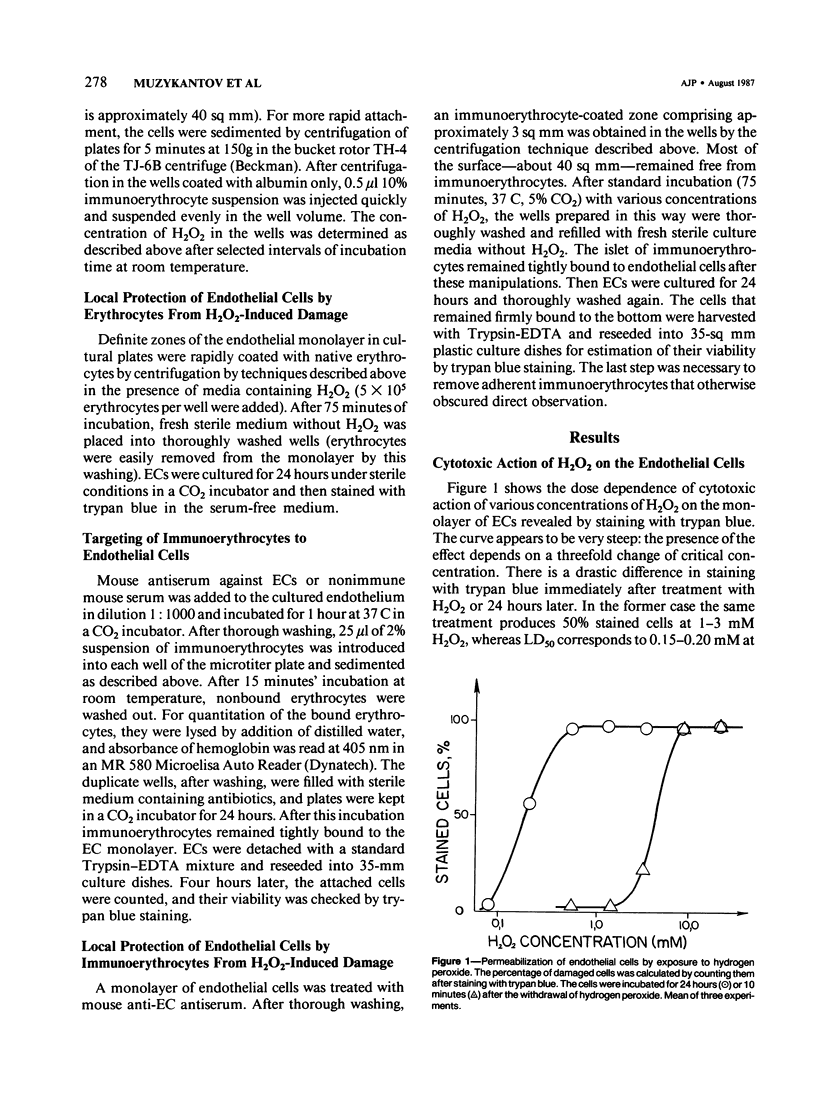
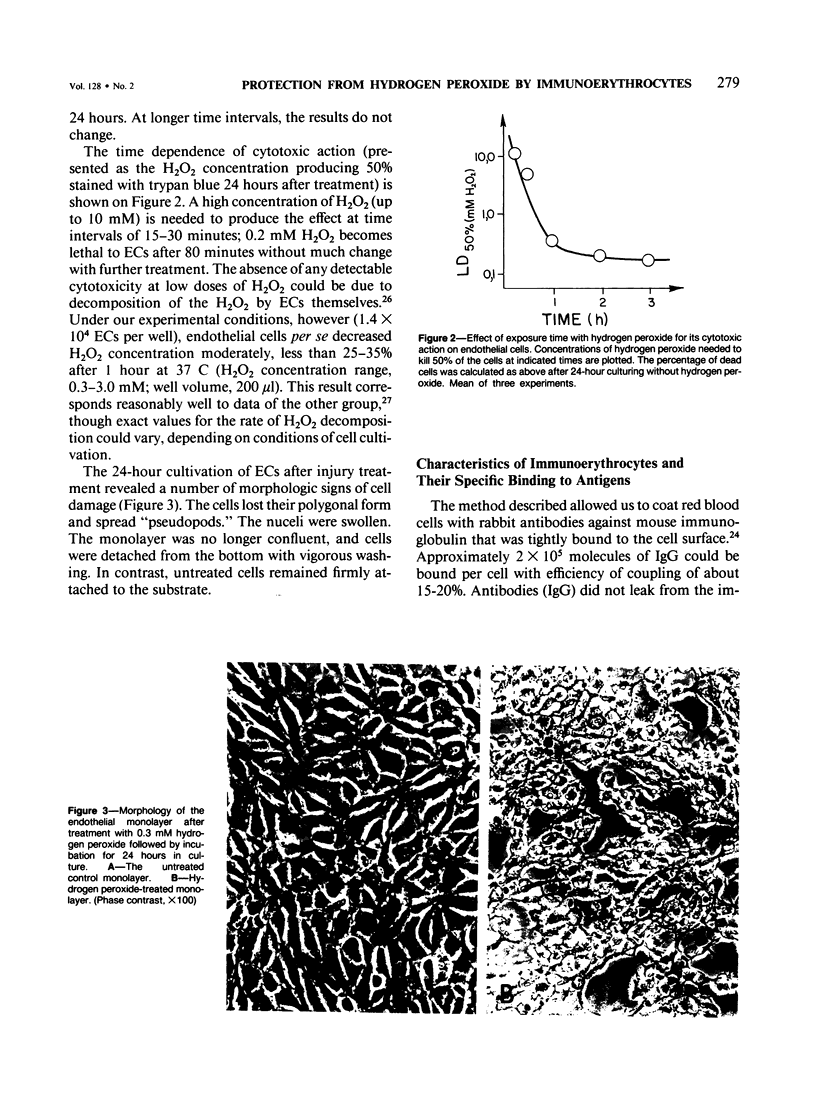
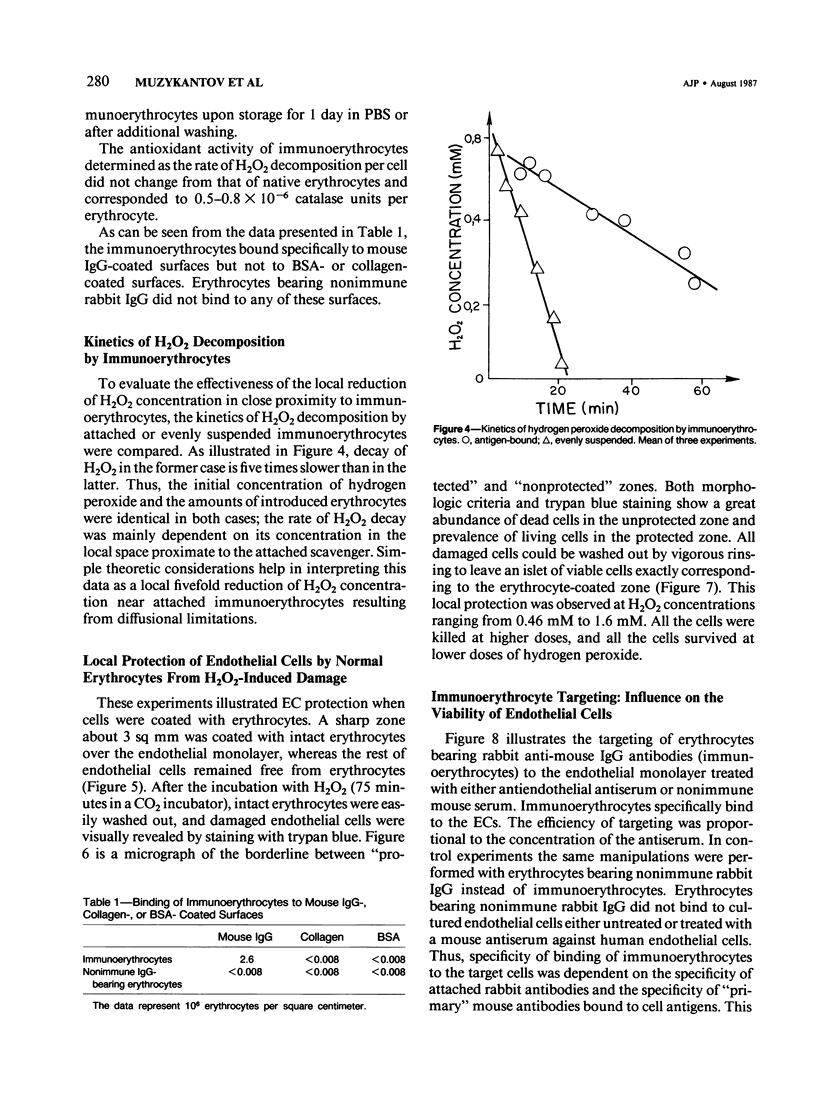
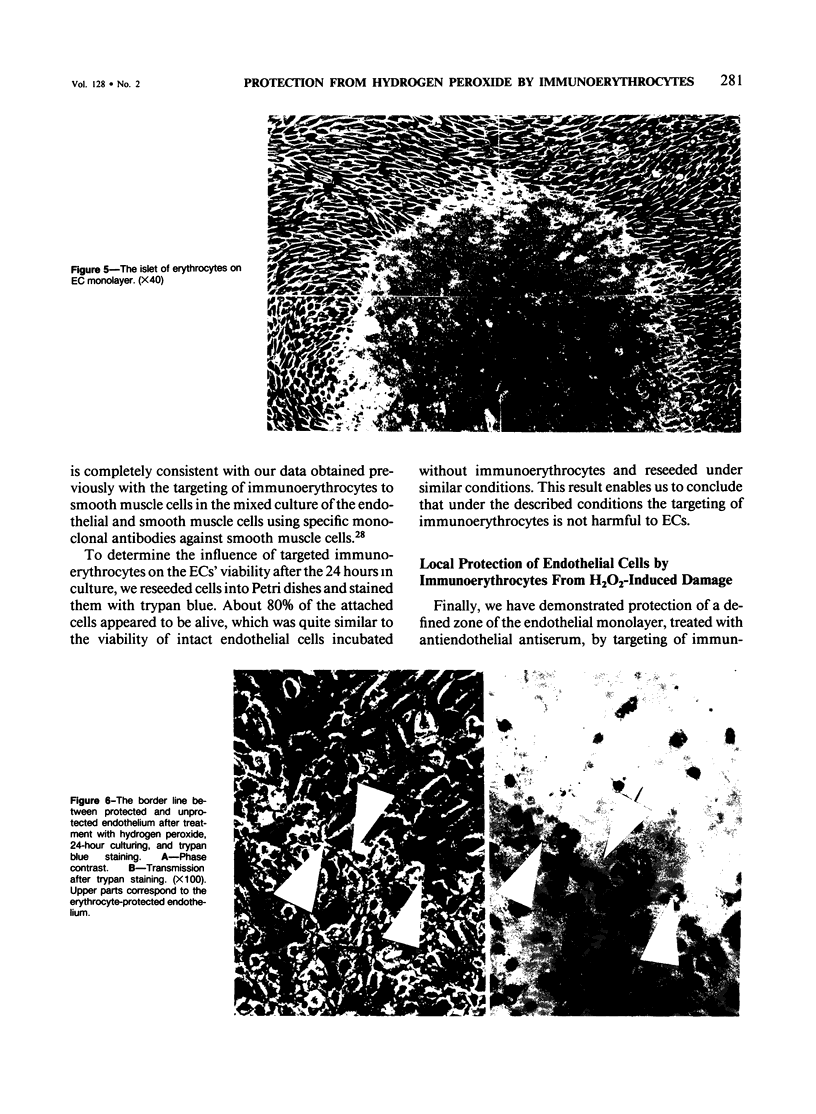
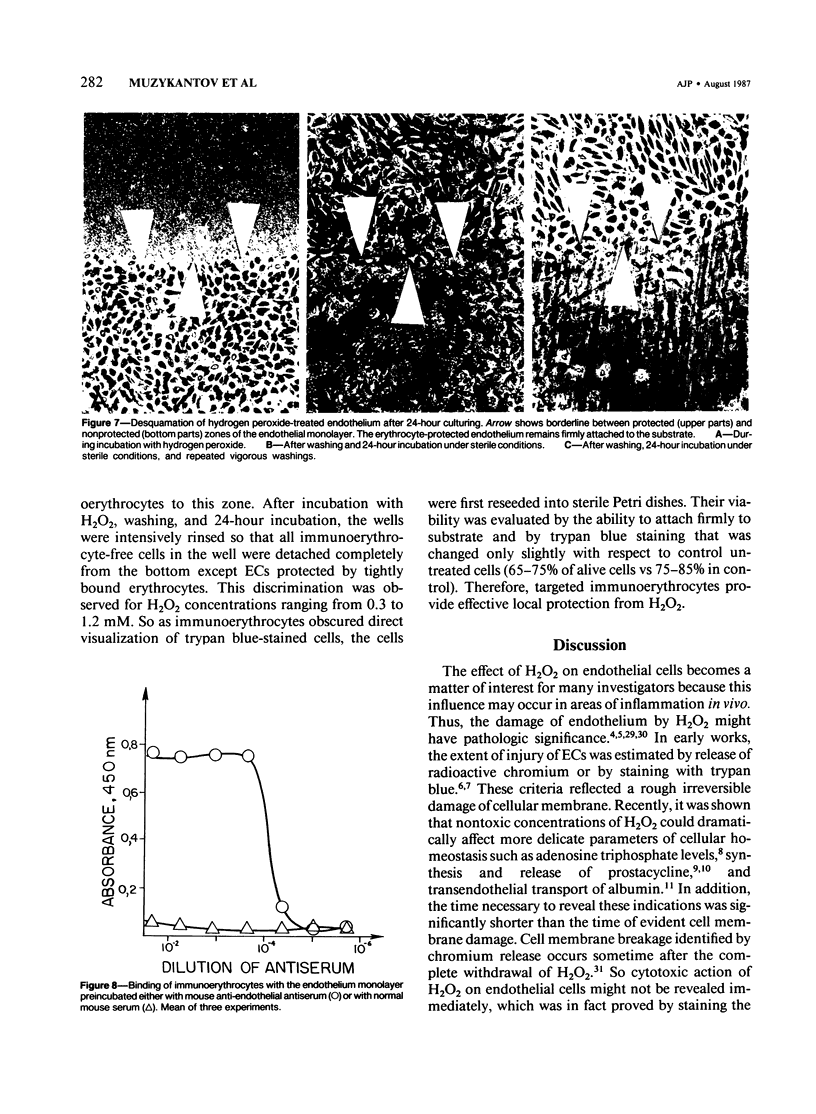
Red blood cells bearing anti-mouse IgG antibody on their surface (immunoerythrocytes) may provide for local protection of endothelial cells from the action of hydrogen peroxide. Subconfluent cultures of human umbilical vein endothelial cells responded sharply to increasing concentrations of hydrogen peroxide. Permeabilization of cellular membrane occurred at doses of hydrogen peroxide of from 1 to 3 mM, and was assured by incorporation of trypan blue stain immediately after treatment. Latent damage of cells produced by much lower doses of hydrogen peroxide (0.2-0.4 mM) could be observed after 24-hour incubation of treated cells in the normal culture medium with no hydrogen peroxide. The apparently dead cells differed from intact cells in morphology, were poorly attached to the substrate, and were readily incorporated by trypan blue, thus permitting easy visualization. Immunoerythrocytes bound to the antigen-coated surface enzymatically decreased the concentration of hydrogen peroxide in their microenvironment at least fivefold with respect to the total hydrogen peroxide concentration. Erythrocytes deposited on a part of the endothelial monolayer locally protected it from the damage at hydrogen peroxide concentrations ranging from 0.4 to 1.2 mM. Localization of protected zones corresponded precisely to the geometry of the erythrocyte coating. Immunoerythrocytes targeted to the endothelial cells by means of mouse anti-endothelial antiserum did not impair their viability and protected the endothelium from being killed at 0.3-1.2 mM hydrogen peroxide. This approach might be useful for a cell selection in mixed cell populations. The problem of local protection of cells involved in the inflammation focus are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agar N. S., Sadrzadeh S. M., Hallaway P. E., Eaton J. W. Erythrocyte catalase. A somatic oxidant defense? J Clin Invest. 1986 Jan;77(1):319–321. doi: 10.1172/JCI112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Granulocyte-dependent injury of pulmonary endothelium: a case of miscommunication? Tissue Cell. 1984;16(2):137–155. doi: 10.1016/0040-8166(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrina A., Patriarca P. Neutrophil-endothelial cell interaction. Evidence for and mechanisms of the self-protection of bovine microvascular endothelial cells from hydrogen peroxide-induced oxidative stress. J Clin Invest. 1986 Aug;78(2):462–471. doi: 10.1172/JCI112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Ward P. A., Johnson K. J., Till G. O. Evidence for a role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984 Jun;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Turrens J. F., Mirza Z., Crapo J. D., Young S. L. Modulation of oxidant lung injury by using liposome-entrapped superoxide dismutase and catalase. Fed Proc. 1985 Jul;44(10):2591–2595. [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Glukhova M. A., Domogatsky S. P., Kabakov A. E., Muzykantov V. R., Ornatsky O. I., Sakharov D. V., Frid M. G., Smirnov V. N. Red blood cell targeting to smooth muscle cells. FEBS Lett. 1986 Mar 17;198(1):155–158. doi: 10.1016/0014-5793(86)81203-0. [DOI] [PubMed] [Google Scholar]
- Hammond B., Kontos H. A., Hess M. L. Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury, and in cerebral vascular damage. Can J Physiol Pharmacol. 1985 Mar;63(3):173–187. doi: 10.1139/y85-034. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Huang A., Kennel S. J., Huang L. Interactions of immunoliposomes with target cells. J Biol Chem. 1983 Nov 25;258(22):14034–14040. [PubMed] [Google Scholar]
- Kirkpatrick C. J., Bültmann B. D., Gruler H. Interaction between enteroviruses and human endothelial cells in vitro. Alterations in the physical properties of endothelial cell plasma membrane and adhesion of human granulocytes. Am J Pathol. 1985 Jan;118(1):15–25. [PMC free article] [PubMed] [Google Scholar]
- Lakow E., Basch R. S. Positive immunoselection using antibody-enzyme complexes. J Immunol Methods. 1981;44(2):135–151. doi: 10.1016/0022-1759(81)90341-0. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R., Sakharov D. V., Smirnov M. D., Domogatsky S. P., Samokhin G. P. Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface. FEBS Lett. 1985 Mar 11;182(1):62–66. doi: 10.1016/0014-5793(85)81154-6. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cohn Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med. 1980 Jul 1;152(1):198–208. doi: 10.1084/jem.152.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznansky M. J., Juliano R. L. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev. 1984 Dec;36(4):277–336. [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Wiggins R. C., Kunkel R. G., Ward P. A. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest. 1984 Oct;51(4):396–403. [PubMed] [Google Scholar]
- Romagnani S., Almerigogna F., Giudizi G. M., Ricci M. Rosette formation with protein A-coated erythrocytes: a method for detecting both IgG-bearing cells and another subset of human peripheral blood B lymphocytes. J Immunol Methods. 1980;33(1):11–21. doi: 10.1016/0022-1759(80)90078-2. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Simon R. H., DeHart P. D., Todd R. F., 3rd Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J Clin Invest. 1986 Nov;78(5):1375–1386. doi: 10.1172/JCI112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Danis E. H., Del Vecchio P. J., Rosano C. L. Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun. 1985 Feb 28;127(1):270–276. doi: 10.1016/s0006-291x(85)80154-6. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Vissers M. C., Day W. A., Winterbourn C. C. Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachment. Blood. 1985 Jul;66(1):161–166. [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Hatherill J. R., Annesley T. M., Kunkel R. G. Systemic complement activation, lung injury, and products of lipid peroxidation. J Clin Invest. 1985 Aug;76(2):517–527. doi: 10.1172/JCI112001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck B. S., Hoidal J., Vercellotti G. M., Schwartz B. A., Moldow C. F., Jacob H. S. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science. 1985 Feb 15;227(4688):756–759. doi: 10.1126/science.2982213. [DOI] [PubMed] [Google Scholar]






