Abstract
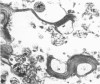
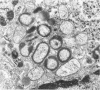
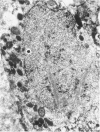
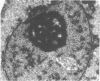
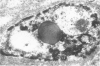
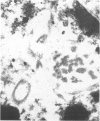
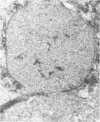
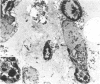
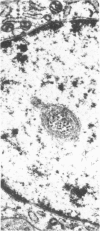
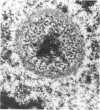
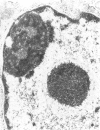
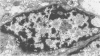
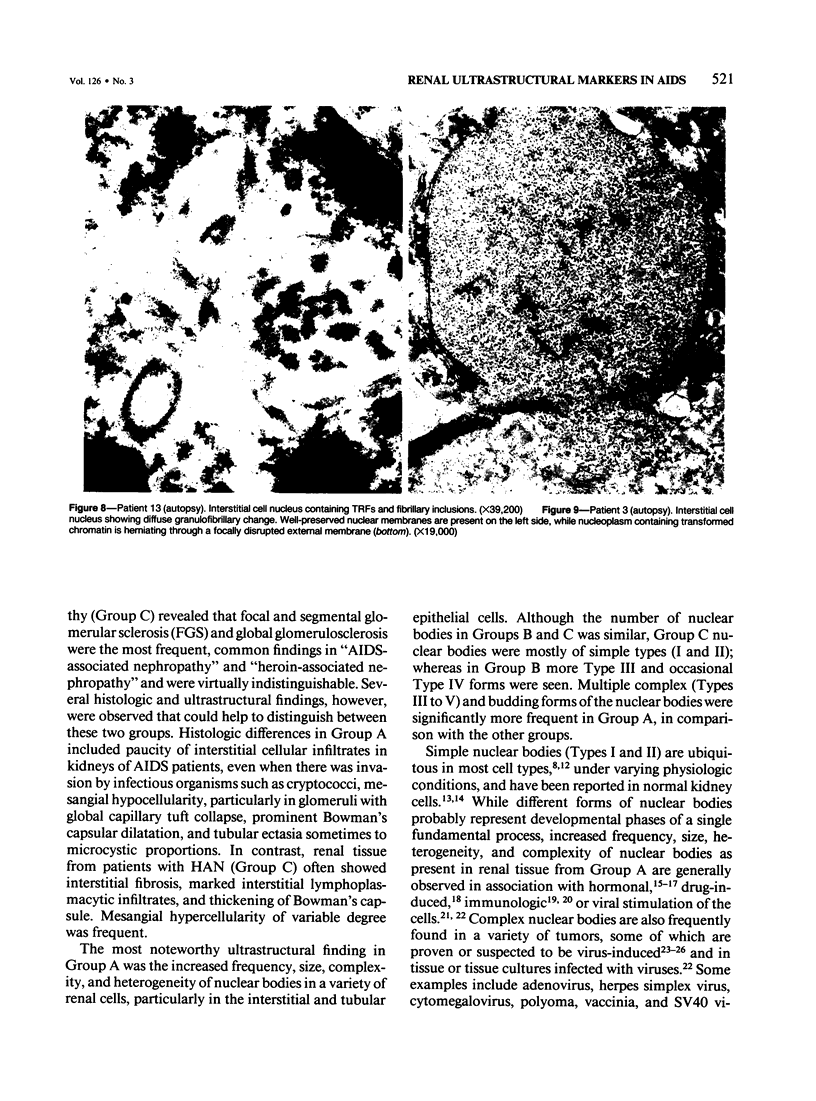
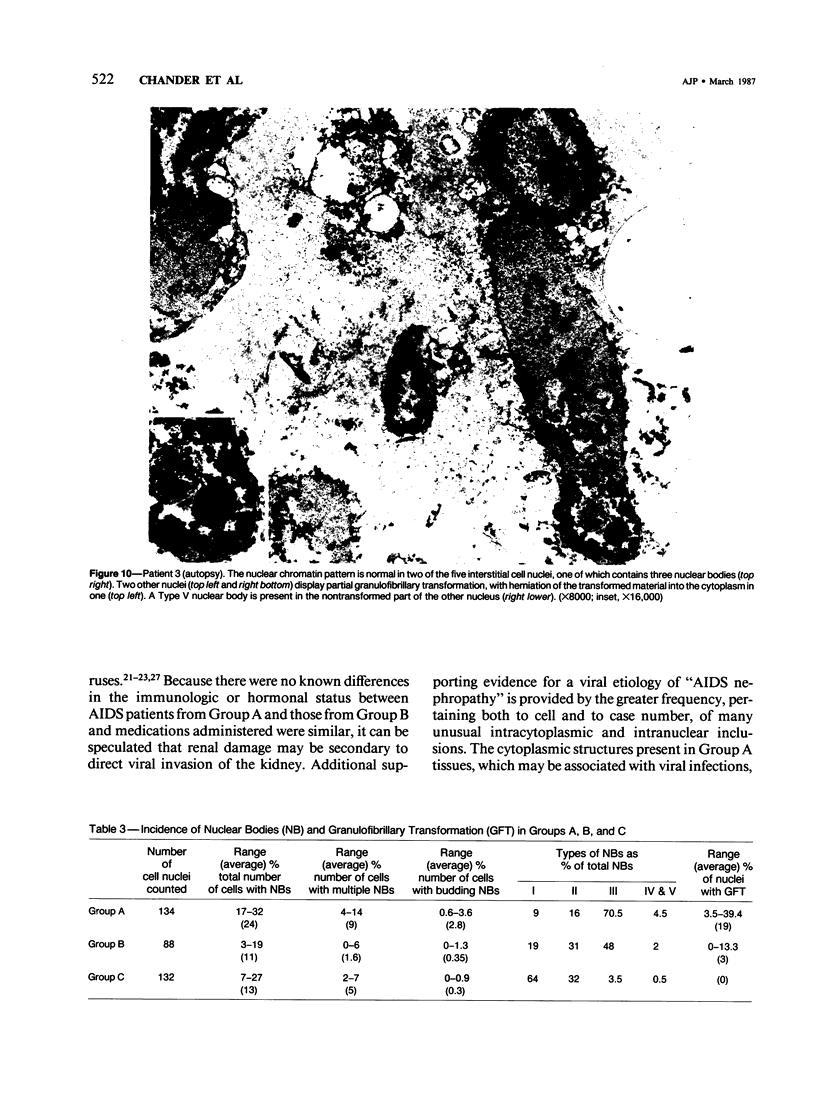
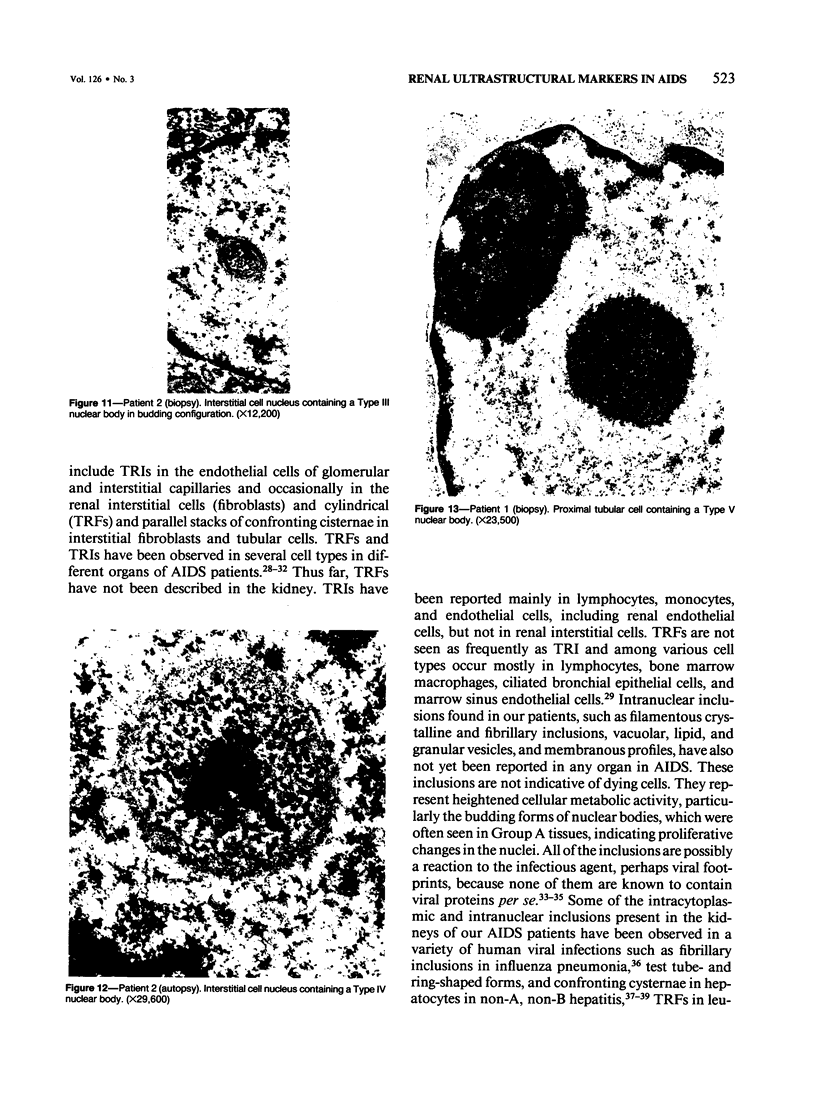
Renal tissues from two groups of patients with acquired immune deficiency syndrome (AIDS) were examined: Group A had severe proteinuria and varying degrees of renal insufficiency, designated AIDS-associated nephropathy (AAN), and Group B had no renal involvement. Control Group C consisted of patients with heroin-associated nephropathy (HAN) with proteinuria comparable to patients in Group A but without AIDS or its related complex (ARC). The most frequent finding, common to both AAN and HAN, was focal glomerular sclerosis. In contrast to HAN, AAN tissue showed mesangial hypocellularity, sparse interstitial infiltrates, severe tubular degenerative changes, tubular microcystic ectasia, Bowman's space dilatation, and presence of multiple complex inclusions both in the nuclei and cytoplasm in a variety of cells. Abundant tubuloreticular inclusions were found in the endothelial and occasionally in the interstitial cell cytoplasm. Nuclear bodies (NBs) were seen in greater frequency, complexity, size, and heterogeneity, and of budding configuration in Group A as compared with Groups B and C; NBs in Group C were mostly of simple types (I and II). In addition, a peculiar granulofibrillary transformation in many tubular and interstitial cell nuclei was observed in Group A. This transformation was rarely present in Group B and was never seen in Group C. Because complex NBs (Types III to V) and various intracytoplasmic and intranuclear inclusions present in Group A are often associated with viral invasion, their presence in kidneys of AIDS patients with proteinuria suggests a viral etiology for AAN.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale M. G., Hoffsten P. E., Robson A. M., MacDermott R. P. Inhibitory factors of lymphocyte transformation in sera from patients with minimal change nephrotic syndrome. Clin Nephrol. 1980 Jun;13(6):271–276. [PubMed] [Google Scholar]
- Bouteille M., Kalifat S. R., Delarue J. Ultrastructural variations of nuclear bodies in human diseases. J Ultrastruct Res. 1967 Aug 30;19(5):474–486. doi: 10.1016/s0022-5320(67)80074-1. [DOI] [PubMed] [Google Scholar]
- Dahl E. Studies of the fine structure of ovarian interstitial tissue. 6. Effects of clomiphene on the thecal gland of the domestic fowl. Z Zellforsch Mikrosk Anat. 1970;109(2):227–244. doi: 10.1007/BF00365243. [DOI] [PubMed] [Google Scholar]
- Dumont A., Robert A. Ultrastructure of complex nuclear bodies produced experimentally in hamster peritoneal macrophages. J Ultrastruct Res. 1971 Aug;36(3):483–492. doi: 10.1016/s0022-5320(71)80119-3. [DOI] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Spira T. J., Chandler F. W., Callaway C. S., Brynes R. K., Chan W. C. Unusual cytoplasmic body in lymphoid cells of homosexual men with unexplained lymphadenopathy. A preliminary report. N Engl J Med. 1983 Apr 7;308(14):819–822. doi: 10.1056/NEJM198304073081407. [DOI] [PubMed] [Google Scholar]
- Fodor P., Saitúa M. T., Rodriguez E., González B., Schlesinger L. T-cell dysfunction in minimal-change nephrotic syndrome of childhood. Am J Dis Child. 1982 Aug;136(8):713–717. doi: 10.1001/archpedi.1982.03970440057016. [DOI] [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardenswartz M. H., Lerner C. W., Seligson G. R., Zabetakis P. M., Rotterdam H., Tapper M. L., Michelis M. F., Bruno M. S. Renal disease in patients with AIDS: a clinicopathologic study. Clin Nephrol. 1984 Apr;21(4):197–204. [PubMed] [Google Scholar]
- Gresser I., Aguet M., Morel-Maroger L., Woodrow D., Puvion-Dutilleul F., Guillon J. C., Maury C. Electrophoretically pure mouse interferon inhibits growth, induces liver and kidney lesions, and kills suckling mice. Am J Pathol. 1981 Mar;102(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Maury C., Tovey M., Morel-Maroger L., Pontillon F. Progressive glomerulonephritis in mice treated with interferon preparations at birth. Nature. 1976 Sep 30;263(5576):420–422. doi: 10.1038/263420a0. [DOI] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostianovsky M., Kang Y. H., Grimley P. M. Ultrastructural and immunoelectron microscopic studies of cells with abnormal cytoplasmic inclusions in patients with AIDS. AIDS Res. 1983 1984;1(3):181–196. doi: 10.1089/aid.1.1983.1.181. [DOI] [PubMed] [Google Scholar]
- Krishan A., Uzman B. G., Hedley-Whyte E. T. Nuclear bodies: a component of cell nuclei in hamster tissues and human tumors. J Ultrastruct Res. 1967 Aug 30;19(5):563–572. doi: 10.1016/s0022-5320(67)80082-0. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. Relations of the centrolobular region of the glomerulus to the juxtaglomerular apparatus. J Ultrastruct Res. 1962 Jun;6:562–578. doi: 10.1016/s0022-5320(62)80010-0. [DOI] [PubMed] [Google Scholar]
- Lafontaine J. G. A light and electron microscope study of small, spherical nuclear bodies in meristematic cells of Allium cepa, Vicia faba, and Raphanus sativus. J Cell Biol. 1965 Jul;26(1):1–17. doi: 10.1083/jcb.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrue G., Xheneumont S., Branellec A., Hirbec G., Weil B. A vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine. 1975 Feb 10;23(1):37–40. [PubMed] [Google Scholar]
- Levy J. A., Kaminsky L. S., Morrow W. J., Steimer K., Luciw P., Dina D., Hoxie J., Oshiro L. Infection by the retrovirus associated with the acquired immunodeficiency syndrome. Clinical, biological, and molecular features. Ann Intern Med. 1985 Nov;103(5):694–699. doi: 10.7326/0003-4819-103-5-694. [DOI] [PubMed] [Google Scholar]
- Mallick N. P., Williams R. J., McFarlane H., Orr W. M., Taylor G., Williams G. Cell-mediated immunity in nephrotic syndrome. Lancet. 1972 Mar 4;1(7749):507–509. doi: 10.1016/s0140-6736(72)90174-2. [DOI] [PubMed] [Google Scholar]
- Moorthy A. V., Zimmerman S. W., Burkholder P. M. Inhibition of lymphocyte blastogenesis by plasma of patients with minimal-change nephrotic syndrome. Lancet. 1976 May 29;1(7970):1160–1162. doi: 10.1016/s0140-6736(76)91545-2. [DOI] [PubMed] [Google Scholar]
- Pardo V., Aldana M., Colton R. M., Fischl M. A., Jaffe D., Moskowitz L., Hensley G. T., Bourgoignie J. J. Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Oct;101(4):429–434. doi: 10.7326/0003-4819-101-4-429. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Middelkamp J. N. In vivo and in vitro demonstration of nuclear bodies in vaccinia infected cells. J Ultrastruct Res. 1969 Aug;28(3):275–287. doi: 10.1016/s0022-5320(69)90085-9. [DOI] [PubMed] [Google Scholar]
- Pfeifer U., Thomssen R., Legler K., Böttcher U., Gerlich W., Weinmann E., Klinge O. Experimental non-A, non-B hepatitis: four types of cytoplasmic alteration in hepatocytes of infected chimpanzees. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(3):233–243. doi: 10.1007/BF02899184. [DOI] [PubMed] [Google Scholar]
- Rao T. K., Filippone E. J., Nicastri A. D., Landesman S. H., Frank E., Chen C. K., Friedman E. A. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Mar 15;310(11):669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- Rich S. A. Human lupus inclusions and interferon. Science. 1981 Aug 14;213(4509):772–775. doi: 10.1126/science.6166984. [DOI] [PubMed] [Google Scholar]
- Ronco P., Woodrow D., Rivière Y., Moss J., Verroust P., Guillon J. C., Gresser I., Sloper J. C., Morel-Maroger L. Further studies on the inhibition of lymphocytic choriomeningitis-induced glomerulonephritis by antiinterferon globulin. Circulating immune complexes and ultrastructural studies. Lab Invest. 1980 Jul;43(1):37–46. [PubMed] [Google Scholar]
- Ryder D. R., Horvath E., Kovacs K. Nuclear inclusions in the human adenohypophysis. A fine-structural study of nontumorous and adenomatous pituitaries. Acta Anat (Basel) 1979;105(3):273–283. [PubMed] [Google Scholar]
- Safai B., Good R. A. Kaposi's sarcoma: a review and recent developments. CA Cancer J Clin. 1981 Jan-Feb;31(1):2–12. doi: 10.3322/canjclin.31.1.2. [DOI] [PubMed] [Google Scholar]
- Santibañez G. P., Lafarga M. Nuclear bodies in the rat adrenal glomerular zone in normal and experimental conditions. Z Mikrosk Anat Forsch. 1979;93(5):951–958. [PubMed] [Google Scholar]
- Schaff Z., Gerety R. J., Grimley P. M., Iwarson S. A., Jackson D. R., Tabor E. Ultrastructural and cytochemical study of hepatocytes and lymphocytes during experimental non-A, non-B infections in chimpanzees. J Exp Pathol. 1985 Spring;2(1):25–36. [PubMed] [Google Scholar]
- Schulze C. Giant nuclear bodies (sphaeridia) in Sertoli cells of patients with testicular tumors. J Ultrastruct Res. 1979 Jun;67(3):267–275. doi: 10.1016/s0022-5320(79)80027-1. [DOI] [PubMed] [Google Scholar]
- Shamoto M., Murakami S., Zenke T. Adult T-cell leukemia in Japan: an ultrastructural study. Cancer. 1981 Apr 1;47(7):1804–1811. doi: 10.1002/1097-0142(19810401)47:7<1804::aid-cncr2820470714>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Feinstone S. M., Purcell R. H., Alter H. J., London W. T. Non-A, non-B hepatitis: ultrastructural evidence for two agents in experimentally infected chimpanzees. Science. 1979 Jul 13;205(4402):197–200. doi: 10.1126/science.451589. [DOI] [PubMed] [Google Scholar]
- Sidhu G. S., Stahl R. E., El-Sadr W., Zolla-Pazner S. Ultrastructural markers of AIDS. Lancet. 1983 Apr 30;1(8331):990–991. doi: 10.1016/s0140-6736(83)92114-1. [DOI] [PubMed] [Google Scholar]
- Sidhu G. S., Stahl R. E., el-Sadr W., Cassai N. D., Forrester E. M., Zolla-Pazner S. The acquired immunodeficiency syndrome: an ultrastructural study. Hum Pathol. 1985 Apr;16(4):377–386. doi: 10.1016/s0046-8177(85)80231-8. [DOI] [PubMed] [Google Scholar]
- Simar L. J. Ultrastructure et constitution des corps nucléaires dans les plasmocytes. Z Zellforsch Mikrosk Anat. 1969;99(2):235–251. [PubMed] [Google Scholar]
- Simard R. Specific nuclear and nucleolar ultrastructural lesions induced by proflavin and similarly acting antimetabolites in tissue culture. Cancer Res. 1966 Nov;26(11):2316–2328. [PubMed] [Google Scholar]
- Takeichi S., Otsuka H., Kimura S. Studies on tumors produced by cells transformed with herpes simplex virus type 2. Gan. 1977 Oct;68(5):653–661. [PubMed] [Google Scholar]
- Tamura H., Aronson B. E. Intranuclear fibrillary inclusions in influenza pneumonia. Arch Pathol Lab Med. 1978 May;102(5):252–257. [PubMed] [Google Scholar]
- Ultrastructural markers in AIDS. Lancet. 1983 Jul 30;2(8344):284–284. [PubMed] [Google Scholar]
- Vagner-Capodano A. M., Mauchamp J., Stahl A., Lissitzky S. Nucleolar budding and formation of nuclear bodies in cultured thyroid cells stimulated by thyrotropin, dibutyryl cyclic AMP, and prostaglandin E2. J Ultrastruct Res. 1980 Jan;70(1):37–51. doi: 10.1016/s0022-5320(80)90020-9. [DOI] [PubMed] [Google Scholar]
- Vaziri N. D., Barbari A., Licorish K., Cesario T., Gupta S. Spectrum of renal abnormalities in acquired immune-deficiency syndrome. J Natl Med Assoc. 1985 May;77(5):369–375. [PMC free article] [PubMed] [Google Scholar]
- Vázquez J. J., Ortuño G., Cervós-Navarro J. An ultrastructural study of spheroidal nuclear bodies found in gliomas. Virchows Arch B Cell Pathol. 1970;5(4):288–293. doi: 10.1007/BF02893570. [DOI] [PubMed] [Google Scholar]
- WEBER A. F., FROMMES S. P. NUCLEAR BODIES: THEIR PREVALENCE, LOCATION, AND ULTRASTRUCTURE IN THE CALF. Science. 1963 Sep 6;141(3584):912–913. doi: 10.1126/science.141.3584.912. [DOI] [PubMed] [Google Scholar]
- WEBER A., WHIPP S., USENIK E., FROMMES S. STRUCTURAL CHANGES IN THE NUCLEAR BODY IN THE ADRENAL ZONA FASCICULATA OF THE CALF FOLLOWING THE ADMINISTRATION OF ACTH. J Ultrastruct Res. 1964 Dec;11:564–576. doi: 10.1016/s0022-5320(64)80082-4. [DOI] [PubMed] [Google Scholar]
- de Jong P. J., Valderrama G., Spigland I., Horwitz M. S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983 Jun 11;1(8337):1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]