Abstract
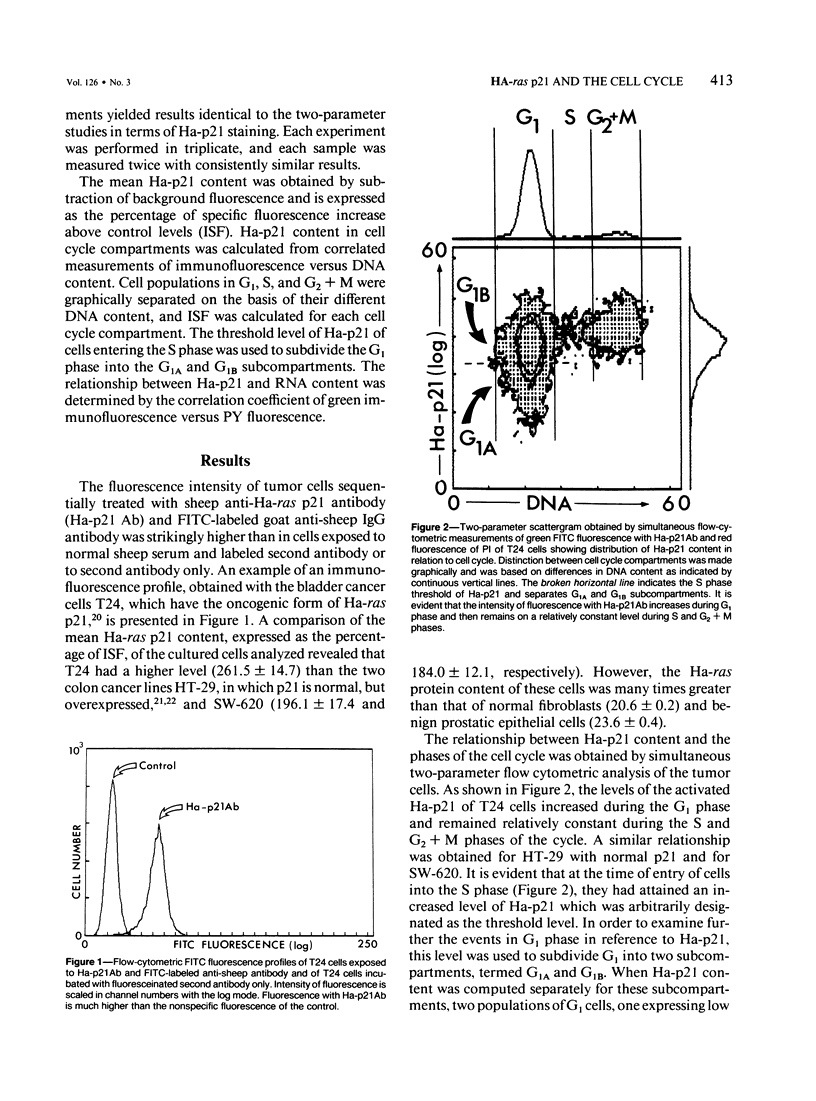
It has been postulated that the expression of the product (p21) encoded by the ras genes may have a role in cell cycle events. Simultaneous multiparameter flow cytometry was used to measure the p21 content in relation to the cell cycle of several cancer cell lines of human origin. These studies revealed that p21 levels rise during the G1 phase of the cycle and remain approximately constant as cells traverse the S and G2 + M phases. The threshold level of p21 expression of S phase cells was used to divide the G1 cell population into cells with low (G1A) and high (G1B) p21 content. The p21 levels of G1B cells were approximately ten times higher than those of G1A cells. The validity of this subdivision was confirmed by synchronous measurements of RNA content and p21. Cells with low RNA content, hence in early part of G1 phase of the cell cycle, expressed low levels of p21, and cells with higher RNA content expressed higher levels of p21. These observations suggest that the levels of p21 are much lower at the onset of the cell cycle than at its end; hence a drop in p21 expression is likely to occur during or immediately after mitotic division.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Bauer K. D., Epstein A. L. A method for simultaneous nuclear immunofluorescence and DNA content quantitation using monoclonal antibodies and flow cytometry. Cytometry. 1985 May;6(3):208–214. doi: 10.1002/cyto.990060306. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Darzynkiewicz Z., Tobey R. A., Steinkamp J. A. Correlated measurements of DNA, RNA, and protein in individual cells by flow cytometry. Science. 1985 Jun 14;228(4705):1321–1324. doi: 10.1126/science.2408339. [DOI] [PubMed] [Google Scholar]
- Czerniak B., Darzynkiewicz Z., Staiano-Coico L., Herz F., Koss L. G. Expression of Ca antigen in relation to cell cycle in cultured human tumor cells. Cancer Res. 1984 Oct;44(10):4342–4346. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Crissman H., Traganos F., Steinkamp J. Cell heterogeneity during the cell cycle. J Cell Physiol. 1982 Dec;113(3):465–474. doi: 10.1002/jcp.1041130316. [DOI] [PubMed] [Google Scholar]
- Durkin J. P., Whitfield J. F. Characterization of G1 transit induced by the mitogenic-oncogenic viral Ki-ras gene product. Mol Cell Biol. 1986 May;6(5):1386–1392. doi: 10.1128/mcb.6.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz F., Schermer A., Halwer M., Bogart L. H. Alkaline phosphatase in HT-29, a human colon cancer cell line: influence of sodium butyrate and hyperosmolality. Arch Biochem Biophys. 1981 Sep;210(2):581–591. doi: 10.1016/0003-9861(81)90224-1. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. Protooncogene expression during the cell cycle. Lab Invest. 1986 Apr;54(4):365–376. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Smith M. R., Bekesi E., Manne V., Stacey D. W. Reversal of transformed phenotype by monoclonal antibodies against Ha-ras p21 proteins. Exp Cell Res. 1986 Feb;162(2):363–371. doi: 10.1016/0014-4827(86)90341-1. [DOI] [PubMed] [Google Scholar]
- Liu H. T., Baserga R., Mercer W. E. Adenovirus type 2 activates cell cycle-dependent genes that are a subset of those activated by serum. Mol Cell Biol. 1985 Nov;5(11):2936–2942. doi: 10.1128/mcb.5.11.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy J., Grimson R., Mishriki Y., Chao S., Oravez S., Fromowitz F., Viola M. V. Elevated ras oncogene expression correlates with lymph node metastases in breast cancer patients. J Clin Oncol. 1986 Sep;4(9):1321–1325. doi: 10.1200/JCO.1986.4.9.1321. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Shapiro H. M. Flow cytometric estimation of DNA and RNA content in intact cells stained with Hoechst 33342 and pyronin Y. Cytometry. 1981 Nov;2(3):143–150. doi: 10.1002/cyto.990020302. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O. Oncogenes and cancer: the p21 ras genes. Cancer Invest. 1984;2(2):109–123. doi: 10.3109/07357908409020294. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tahara E., Yasui W., Taniyama K., Ochiai A., Yamamoto T., Nakajo S., Yamamoto M. Ha-ras oncogene product in human gastric carcinoma: correlation with invasiveness, metastasis or prognosis. Jpn J Cancer Res. 1986 Jun;77(6):517–522. [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Cline M. J. Efficient generation of antibodies to oncoproteins by using synthetic peptide antigens. Proc Natl Acad Sci U S A. 1985 May;82(10):3400–3404. doi: 10.1073/pnas.82.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor A., Horan Hand P., Wunderlich D., Caruso A., Muraro R., Schlom J. Monoclonal antibodies define differential ras gene expression in malignant and benign colonic diseases. Nature. 1984 Oct 11;311(5986):562–565. doi: 10.1038/311562a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Finkel G., Lundy J., Hand P., Thor A., Schlom J. Expression of ras oncogene p21 in prostate cancer. N Engl J Med. 1986 Jan 16;314(3):133–137. doi: 10.1056/NEJM198601163140301. [DOI] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Schlom J. ras Oncogene p21 expression is increased in premalignant lesions and high grade bladder carcinoma. J Exp Med. 1985 May 1;161(5):1213–1218. doi: 10.1084/jem.161.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Pardue R. L., Junker J. L., Takahashi K., Shih T. Y., Weislow O. S. Immunocytochemical localization of RasHa p21 in normal and neoplastic cells in fixed tissue sections from Harvey sarcoma virus-infected mice. Carcinogenesis. 1986 Apr;7(4):645–651. doi: 10.1093/carcin/7.4.645. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]



