Abstract
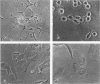
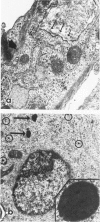
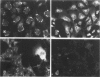
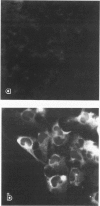
Differentiation of human neuroblastoma (NB) was studied in vitro with five NB cell lines treated with dibutyryl cyclic adenosinemonophosphate and retinoic acid. Although the above agents induced different responses in the various cell lines, three overall morphologic phenotypes emerged: a neuronal, characterized by cell processes and neurosecretory granules, a flat cell without pigment, which displayed basal lamina pertinent to Schwann cells, and a flat pigmented cell which exhibited melanosomes, similarly to melanocytes. The activity of the Schwann cell enzyme cyclic nucleotidyl phosphohydrolase increased considerably in one condition, after induction of a predominantly flat cell phenotype. All studied NB cell lines were capable of synthesizing and expressing the extracellular matrix proteins laminin (LM), fibronectin (FN), and Type IV collagen; but a specific pattern of expression emerged after differentiation, which was proportional to normal tissue equivalents: neuronal--none; melanocytic--FN only; and Schwann cell--large amounts of FN, LM, and Type IV collagen.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Hochholzer L. Ganglioneuroblastoma of the posterior mediastinum: a clinicopathologic review of 80 cases. Cancer. 1981 Jan 15;47(2):373–381. doi: 10.1002/1097-0142(19810115)47:2<373::aid-cncr2820470227>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Keski-Oja J., Vaheri A. Extracellular matrix proteins characterize human tumor cell lines. Int J Cancer. 1981 Jun 15;27(6):755–761. doi: 10.1002/ijc.2910270605. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Dalakas M. C., Lawrence J. V., Carter L. S. Human schwann cells in tissue culture: histochemical and ultrastructural studies. Arch Neurol. 1980 Jun;37(6):329–337. doi: 10.1001/archneur.1980.00500550031001. [DOI] [PubMed] [Google Scholar]
- Aubert C., Janiaud P., Rouge F., Hansson C., Rorsman H., Rosengren E. Melanogenesis in cultured human neuroblastomas. Ann Clin Res. 1980 Dec;12(6):288–294. [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Boesel C. P., Suhan J. P., Sayers M. P. Melanotic medulloblastoma. Report of a case with ultrastructural findings. J Neuropathol Exp Neurol. 1978 Sep;37(5):531–543. doi: 10.1097/00005072-197809000-00008. [DOI] [PubMed] [Google Scholar]
- Bolande R. P. Spontaneous regression and cytodifferentiation of cancer in early life: the oncogenic grace period. Surv Synth Pathol Res. 1985;4(4):296–311. doi: 10.1159/000156982. [DOI] [PubMed] [Google Scholar]
- Bove K. E., McAdams A. J. Composite ganglioneuroblastoma. An assessment of the significance of histological maturation in neuroblastoma diagnosed beyond infancy. Arch Pathol Lab Med. 1981 Jun;105(6):325–330. [PubMed] [Google Scholar]
- Bronner-Fraser M. Distribution of latex beads and retinal pigment epithelial cells along the ventral neural crest pathway. Dev Biol. 1982 May;91(1):50–63. doi: 10.1016/0012-1606(82)90007-0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Latex beads as probes of a neural crest pathway: effects of laminin, collagen, and surface charge on bead translocation. J Cell Biol. 1984 Jun;98(6):1947–1960. doi: 10.1083/jcb.98.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Biosynthesis and secretion of fibronectin in human melanoma cells. J Cell Biochem. 1983;21(2):129–140. doi: 10.1002/jcb.240210204. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M. Neuron-Schwann cell interaction in basal lamina formation. Dev Biol. 1982 Aug;92(2):449–460. doi: 10.1016/0012-1606(82)90190-7. [DOI] [PubMed] [Google Scholar]
- Carbonetto S., Gruver M. M., Turner D. C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983 Nov;3(11):2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Eldridge C. F., Cornbrooks C. J., Timpl R., Bunge R. P. Biosynthesis of type IV collagen by cultured rat Schwann cells. J Cell Biol. 1983 Aug;97(2):473–479. doi: 10.1083/jcb.97.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard P., Coltey P. Cholinergic traits in the neural crest: acetylcholinesterase in crest cells of the chick embryo. Dev Biol. 1983 Jul;98(1):221–238. doi: 10.1016/0012-1606(83)90351-2. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M., Konigsberg I. R. A clonal approach to the problem of neural crest determination. Dev Biol. 1975 Oct;46(2):262–280. doi: 10.1016/0012-1606(75)90104-9. [DOI] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell L. A., Weston J. A. An analysis of melanogenesis in cultured chick embryo spinal ganglia. Dev Biol. 1970 Aug;22(4):670–697. doi: 10.1016/0012-1606(70)90175-2. [DOI] [PubMed] [Google Scholar]
- Dehner L. P., Sibley R. K., Sauk J. J., Jr, Vickers R. A., Nesbit M. E., Leonard A. S., Waite D. E., Neeley J. E., Ophoven J. Malignant melanotic neuroectodermal tumor of infancy: a clinical, pathologic, ultrastructural and tissue culture study. Cancer. 1979 Apr;43(4):1389–1410. doi: 10.1002/1097-0142(197904)43:4<1389::aid-cncr2820430429>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Thiery J. P. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Dev Biol. 1982 Oct;93(2):308–323. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Everson T. C. Spontaneous regression of cancer. Ann N Y Acad Sci. 1964 Apr 2;114(2):721–735. [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969 Nov;115(3):465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., BURDMAN J. A., JOURNEY L. J. LONG-TERM TISSUE CULTURE OF NEUROBLASTOMAS. II. MORPHOLOGIC EVIDENCE FOR DIFFERENTIATION AND MATURATION. J Natl Cancer Inst. 1964 Jan;32:165–199. [PubMed] [Google Scholar]
- GONZALEZ-ANGULO A., REYES H. A., REYNA A. N. THE ULTRASTRUCTURE OF GANGLIONEUROBLASTOMA. OBSERVATIONS ON NEOPLASTIC GANGLION CELLS. Neurology. 1965 Mar;15:242–252. doi: 10.1212/wnl.15.3.242. [DOI] [PubMed] [Google Scholar]
- Glastris B., Pfeiffer S. E. Mammalian membrane marker enzymes: sensitive assay for 5'-nucleotidase and assay for mammalian 2',3'-cyclic-nucleotide-3'-phosphohydrolase. Methods Enzymol. 1974;32:124–131. doi: 10.1016/0076-6879(74)32015-0. [DOI] [PubMed] [Google Scholar]
- Haussler M., Sidell N., Kelly M., Donaldson C., Altman A., Mangelsdorf D. Specific high-affinity binding and biologic action of retinoic acid in human neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5525–5529. doi: 10.1073/pnas.80.18.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H. J., Marks A., Thom H., Baumal R. Role of antibody to S100 protein in diagnostic pathology. Am J Clin Pathol. 1983 Mar;79(3):341–347. doi: 10.1093/ajcp/79.3.341. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma--origin from a primitive neuroectodermal cell? Nature. 1984 Feb 2;307(5950):471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. Migration and differentiation of neural crest cells. Curr Top Dev Biol. 1980;16:31–85. doi: 10.1016/s0070-2153(08)60153-2. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeyer R. P., Hallman K. O., Hammar S. P., Raisis J. E., Tytus J. S., Bockus D. Melanotic schwannoma. Clinical and ultrastructural studies of three cases with evidence of intracellular melanin synthesis. Am J Surg Pathol. 1979 Feb;3(1):3–10. doi: 10.1097/00000478-197902000-00001. [DOI] [PubMed] [Google Scholar]
- Mudge A. W. Schwann cells induce morphological transformation of sensory neurones in vitro. Nature. 1984 May 24;309(5966):367–369. doi: 10.1038/309367a0. [DOI] [PubMed] [Google Scholar]
- Murray M. R., Stout A. P. Distinctive Characteristics of the Sympathicoblastoma Cultivated in Vitro: A Method for Prompt Diagnosis. Am J Pathol. 1947 May;23(3):429–441. [PMC free article] [PubMed] [Google Scholar]
- Newgreen D., Thiery J. P. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211(2):269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Nichols D. H., Weston J. A. Melanogenesis in cultures of peripheral nervous tissue. I. The origin and prospective fate of cells giving rise to melanocytes. Dev Biol. 1977 Oct 1;60(1):217–225. doi: 10.1016/0012-1606(77)90120-8. [DOI] [PubMed] [Google Scholar]
- Palm S. L., Furcht L. T. Production of laminin and fibronectin by Schwannoma cells: cell-protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983 May;96(5):1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N. Differentiation of neuroblastoma cells in culture. Biol Rev Camb Philos Soc. 1975 May;50(2):129–165. doi: 10.1111/j.1469-185x.1975.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Kumar S. Role of cyclic AMP in differentiation of human neuroblastoma cells in culture. Cancer. 1975 Oct;36(4):1338–1343. doi: 10.1002/1097-0142(197510)36:4<1338::aid-cncr2820360422>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reddy N. B., Askanas V., Engel W. K. Demonstration of 2',3'-cyclic nucleotide 3'-phosphohydrolase in cultured human Schwann cells. J Neurochem. 1982 Sep;39(3):887–889. doi: 10.1111/j.1471-4159.1982.tb07977.x. [DOI] [PubMed] [Google Scholar]
- Reynolds C. P., Frenkel E. P., Smith R. G. Growth characteristics of neuroblastoma in vitro correlate with patient survival. Trans Assoc Am Physicians. 1980;93:203–211. [PubMed] [Google Scholar]
- Richelson E. Stimulation of tyrosine hydroxylase activity in an adrenergic clone of mouse neuroblastoma by dibutyryl cyclic AMP. Nat New Biol. 1973 Apr 11;242(119):175–177. doi: 10.1038/newbio242175a0. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Letourneau P. C., Palm S. L., McCarthy J., Furcht L. T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983 Jul;98(1):212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Romansky S. G., Crocker D. W., Shaw K. N. Ultrastructural studies on neuroblastoma: evaluation of cytodifferentiation and correlation of morphology and biochemical and survival data. Cancer. 1978 Nov;42(5):2392–2398. doi: 10.1002/1097-0142(197811)42:5<2392::aid-cncr2820420540>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Biedler J. L. Presence and regulation of tyrosinase activity in human neuroblastoma cell variants in vitro. Cancer Res. 1985 Apr;45(4):1628–1632. [PubMed] [Google Scholar]
- Schlesinger H. R., Gerson J. M., Moorhead P. S., Maguire H., Hummeler K. Establishment and characterization of human neuroblastoma cell lines. Cancer Res. 1976 Sep;36(9 PT1):3094–3100. [PubMed] [Google Scholar]
- Sidell N., Altman A., Haussler M. R., Seeger R. C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983 Oct;148(1):21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982 Apr;68(4):589–596. [PubMed] [Google Scholar]
- Smith J., Fauquet M., Ziller C., Le Douarin N. M. Acetylcholine synthesis by mesencephalic neural crest cells in the process of migration in vivo. Nature. 1979 Dec 20;282(5741):853–855. doi: 10.1038/282853a0. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Droms K. On neuronal and glial differentiation of a pluripotent stem cell line, RT4-AC: a branch determination. Curr Top Dev Biol. 1986;20:211–221. doi: 10.1016/s0070-2153(08)60665-1. [DOI] [PubMed] [Google Scholar]
- Taxy J. B. Electron microscopy in the diagnosis of neuroblastoma. Arch Pathol Lab Med. 1980 Jul;104(7):355–360. [PubMed] [Google Scholar]
- Triche T. J., Askin F. B. Neuroblastoma and the differential diagnosis of small-, round-, blue-cell tumors. Hum Pathol. 1983 Jul;14(7):569–595. doi: 10.1016/s0046-8177(83)80202-0. [DOI] [PubMed] [Google Scholar]
- Tsokos M., Kyritsis A. P., Chader G. J., Triche T. J. Differentiation of human retinoblastoma in vitro into cell types with characteristics observed in embryonal or mature retina. Am J Pathol. 1986 Jun;123(3):542–552. [PMC free article] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Waymire J. C., Weiner N., Prasad K. N. Regulation of tyrosine hydroxylase activity in cultured mouse neuroblastoma cells: elevation induced by analogs of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2241–2245. doi: 10.1073/pnas.69.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]