Abstract
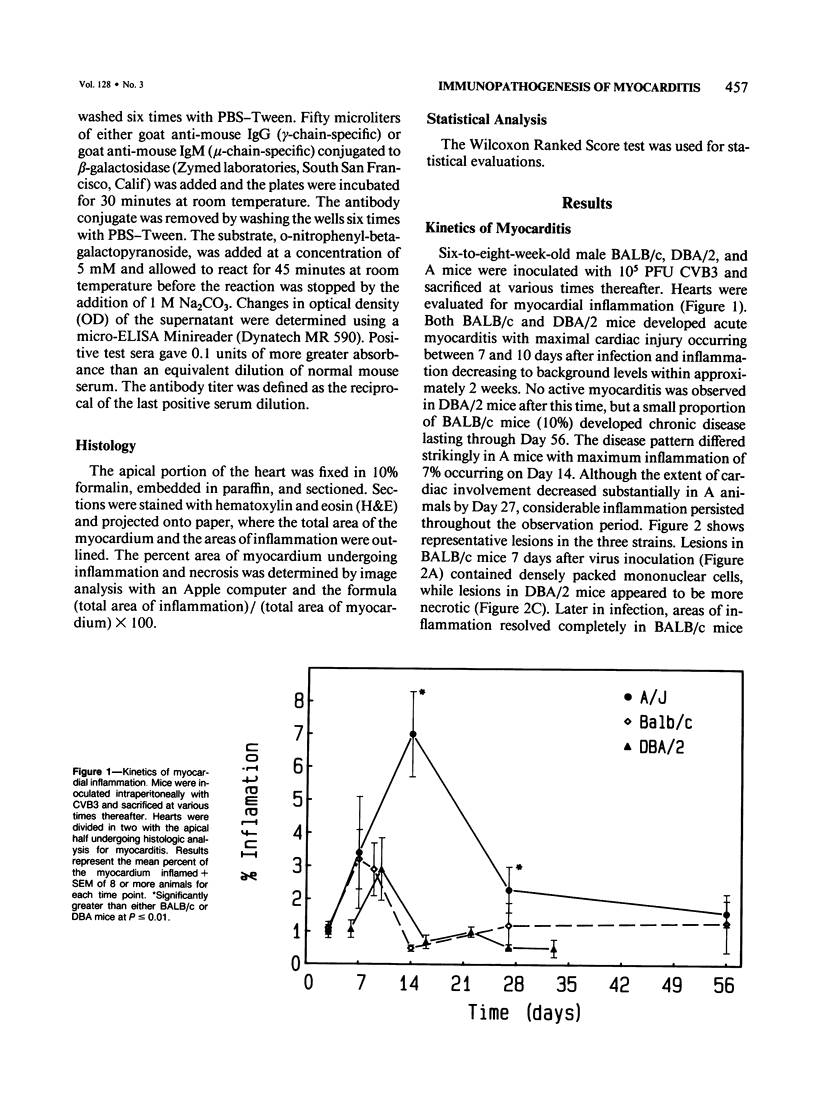
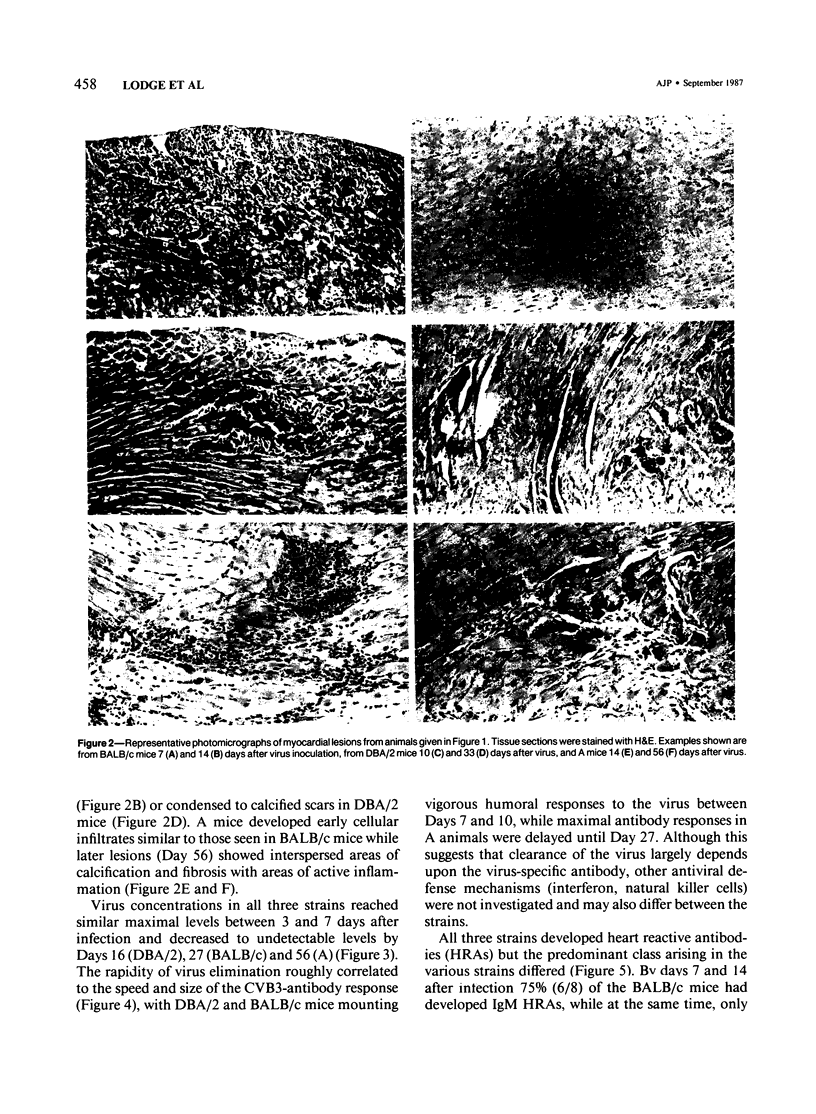
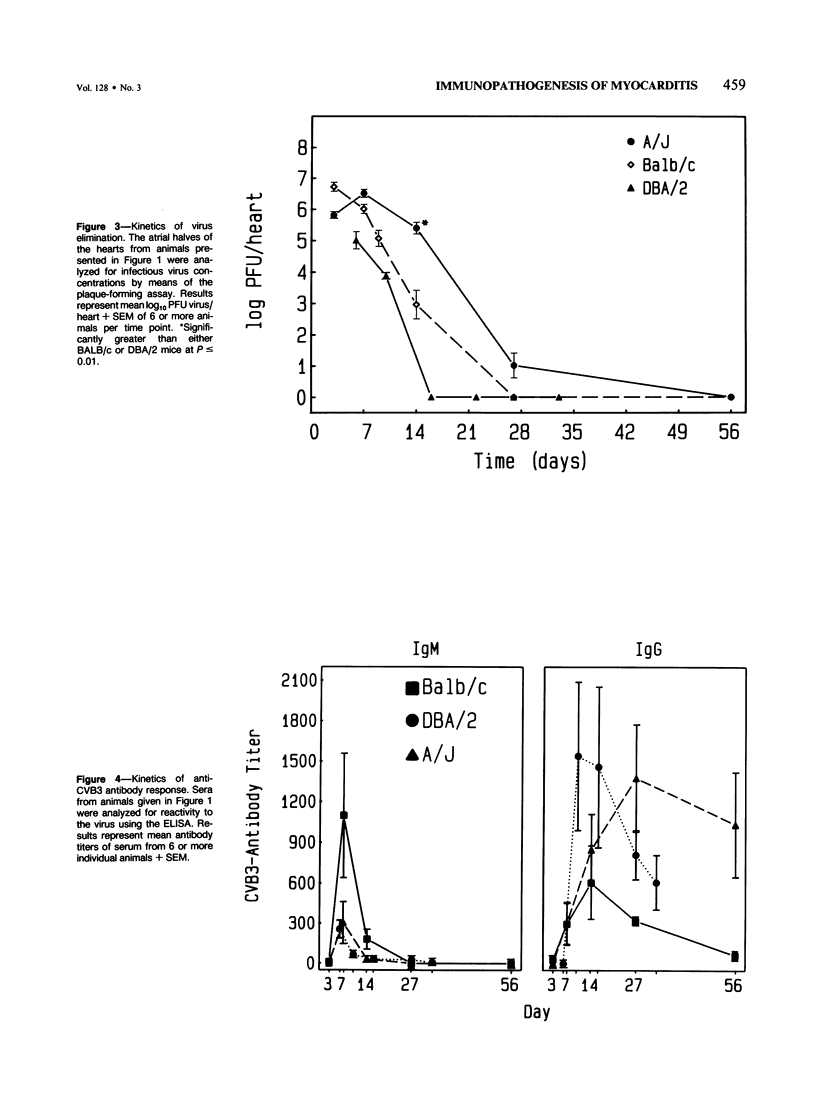
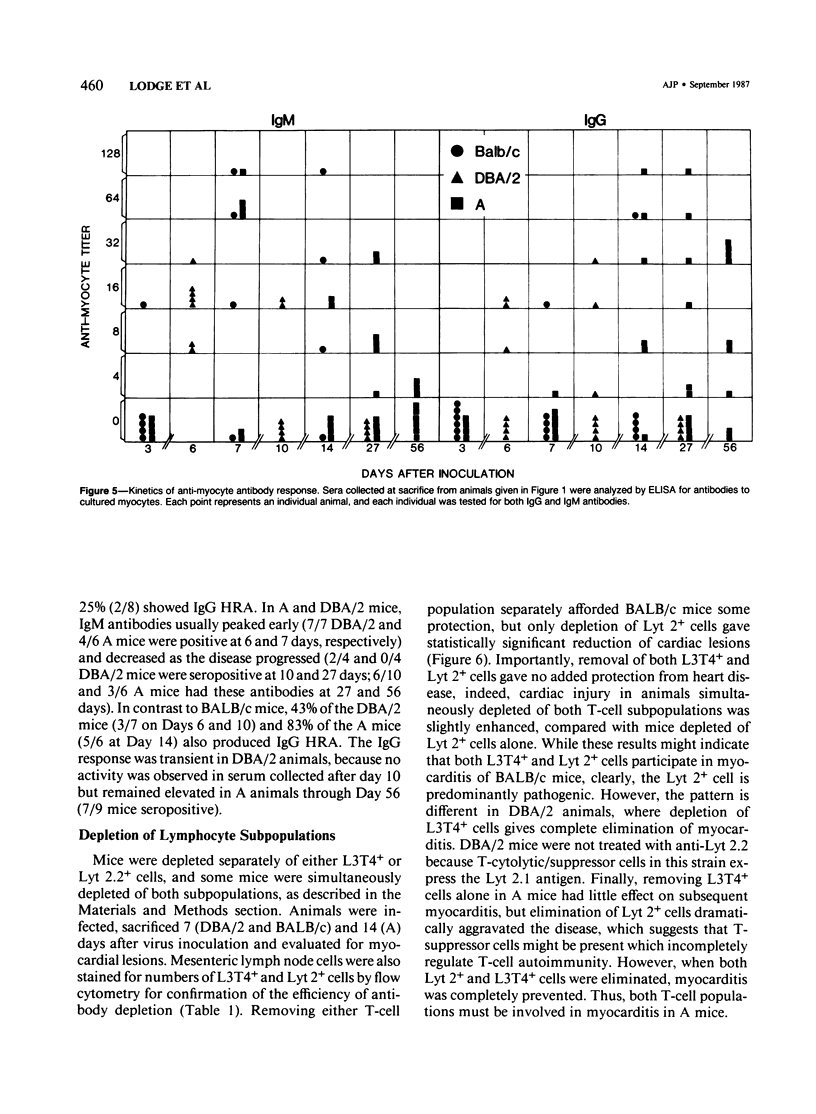
Male BALB/c, DBA/2 and A mice inoculated with a myocarditic variant of coxsackievirus group B, Type 3 (CVB3) developed three distinct patterns of myocarditis. Most BALB/c and all DBA/2 mice developed acute cardiac inflammation lasting only 2 weeks, while A animals consistently showed twice as much myocardial damage as the other two strains, with active myocarditis continuing for 56 days after virus inoculation. Although virus elimination from the heart was also delayed in A mice, immunopathogenic, not viral, mechanisms caused cardiac injury in all strains. In vivo depletion of L3T4+ (T helper) and Lyt 2+ (T cytolytic/suppressor) cells using monoclonal antibodies showed that myocarditis in BALB/c mice depended predominantly on Lyt 2+ cells, in DBA/2 mice on L3T4+ cells and in A mice on both Lyt 2+ and L3T4+ cells. IgM heart reactive antibodies (HRAs) occurred in all three strains, while IgG HRAs were detected only in DBA/2 and A mice. Presumably, only the IgG antibody was pathogenic in this system. These data suggest that the host's genetic makeup determines the type of immune response to CVB3 infection of the heart and therefore the pattern of myocarditis which develops.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Waldmann H. A rapid solid-phase enzyme-linked binding assay for screening monoclonal antibodies to cell surface antigens. J Immunol Methods. 1981;44(2):125–133. doi: 10.1016/0022-1759(81)90340-9. [DOI] [PubMed] [Google Scholar]
- Estrin M., Huber S. A. Coxsackievirus B3-induced myocarditis. Autoimmunity is L3T4+ T helper cell and IL-2 independent in BALB/c mice. Am J Pathol. 1987 May;127(2):335–341. [PMC free article] [PubMed] [Google Scholar]
- Estrin M., Smith C., Huber S. Coxsackievirus B-3 myocarditis. T-cell autoimmunity to heart antigens is resistant to cyclosporin-A treatment. Am J Pathol. 1986 Nov;125(2):244–251. [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. 1986 Sep 1;137(5):1695–1702. [PubMed] [Google Scholar]
- Guthrie M., Lodge P. A., Huber S. A. Cardiac injury in myocarditis induced by Coxsackievirus group B, type 3 in Balb/c mice is mediated by Lyt 2 + cytolytic lymphocytes. Cell Immunol. 1984 Oct 15;88(2):558–567. doi: 10.1016/0008-8749(84)90188-6. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lyden D. C., Lodge P. A. Immunopathogenesis of experimental Coxsackievirus induced myocarditis: role of autoimmunity. Herz. 1985 Feb;10(1):1–7. [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. Coxsackie virus B3 perimyocarditis in BALB/c mice: experimental model of chronic perimyocarditis in the right ventricle. J Pathol. 1980 Jun;131(2):97–106. doi: 10.1002/path.1711310202. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C., Sawada S. Encephalomyocarditis virus myocarditis in inbred strains of mice--chronic stage. Jpn Circ J. 1982 Nov;46(11):1192–1196. doi: 10.1253/jcj.46.1192. [DOI] [PubMed] [Google Scholar]
- Monrad E. S., Matsumori A., Murphy J. C., Fox J. G., Crumpacker C. S., Abelmann W. H. Therapy with cyclosporine in experimental murine myocarditis with encephalomyocarditis virus. Circulation. 1986 May;73(5):1058–1064. doi: 10.1161/01.cir.73.5.1058. [DOI] [PubMed] [Google Scholar]
- O'Connell J. B., Reap E. A., Robinson J. A. The effects of cyclosporine on acute murine Coxsackie B3 myocarditis. Circulation. 1986 Feb;73(2):353–359. doi: 10.1161/01.cir.73.2.353. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Coxsackievirus myocarditis--with special reference to acute and chronic effects. Prog Cardiovasc Dis. 1985 May-Jun;27(6):373–394. doi: 10.1016/0033-0620(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Skamene E., James S. L., Meltzer M. S., Nesbitt M. N. Genetic control of macrophage activation for killing of extracellular targets. J Leukoc Biol. 1984 Jan;35(1):65–69. doi: 10.1002/jlb.35.1.65. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Woodruff J. F. Lack of correlation between neutralizing antibody production and suppression of coxsackievirus B-3 replication in target organs: evidence for involvement of mononuclear inflammatory cells in host defense. J Immunol. 1979 Jul;123(1):31–36. [PubMed] [Google Scholar]



