Abstract
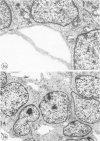
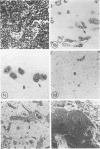
Wilms' tumor has been proposed to originate from a developmental abnormality of the metanephric blastema. This undifferentiated component of Wilms' tumors has previously eluded efforts for in vitro growth. Blastema from a "classical" Wilms' tumor was transplanted into nude mice and passaged through 12 generations of heterotransplantation. Tumors from heterotransplants were grown for 12 serial passages in a serum-free growth medium supplemented with hormones and conditioned media from human kidney proximal tubule cells. The blastema initially grew on a collagen-fetal calf serum matrix as multicellular spheroids, and the cells proliferating from the rim of the spheroids had a flattened shape. Pulse-labeling with bromodeoxyuridine (BrdU) identified the proliferating cell population as blastemal in origin. Except for a loss of extracellular matrix, ultrastructural studies demonstrated morphologic similarities in the cultured cells, compared with the primary tumor and heterotransplants. Lectin histochemical stains for the peanut lectin (PNA) and immunohistochemical stains for cytokeratin (CYTO), vimentin (VIM), and epithelial membrane antigen (EMA) were performed on the original tumor, successive heterotransplants, and cells grown in vitro. The PNA stained the surface of the blastemal cells after sialidase digestion in the original tumor, heterotransplants, and cultured cells. The blastema of the original tumors was negative for CYTO and EMA but reactive for vimentin. This lack of differentiation was maintained in heterotransplants through 12 passages. However, blastemal cells demonstrated coexpression of CYTO and VIM intermediate filaments when grown in a serum-free medium on a matrix material. These studies demonstrate that the blastemal component of Wilms' tumor can be successfully grown in culture, passaged in nude mouse heterotransplants, and shown to undergo early stages of blastemal differentiation in vitro by growth in serum-free medium. This in vitro system provides a model for testing the factors that influence the growth and differentiation of the blastemal component of Wilms' tumors.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Schäfer H., Schauer A., Weber K. Distinction of nephroblastomas from other childhood tumors using antibodies to intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):113–124. doi: 10.1007/BF02889858. [DOI] [PubMed] [Google Scholar]
- Beckwith J. B. Wilms' tumor and other renal tumors of childhood: a selective review from the National Wilms' Tumor Study Pathology Center. Hum Pathol. 1983 Jun;14(6):481–492. doi: 10.1016/s0046-8177(83)80003-3. [DOI] [PubMed] [Google Scholar]
- Detrisac C. J., Sens M. A., Garvin A. J., Spicer S. S., Sens D. A. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984 Feb;25(2):383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- Douglass E. C., Wilimas J. A., Green A. A., Look A. T. Abnormalities of chromosomes 1 and 11 in Wilms' tumor. Cancer Genet Cytogenet. 1985 Jan 15;14(3-4):331–338. doi: 10.1016/0165-4608(85)90199-2. [DOI] [PubMed] [Google Scholar]
- Garvin A. J., Surrette F., Hintz D. S., Rudisill M. T., Sens M. A., Sens D. A. The in vitro growth and characterization of the skeletal muscle component of Wilms' tumor. Am J Pathol. 1985 Nov;121(2):298–310. [PMC free article] [PubMed] [Google Scholar]
- Hennigar R. A., Schulte B. A., Spicer S. S. Heterogeneous distribution of glycoconjugates in human kidney tubules. Anat Rec. 1985 Apr;211(4):376–390. doi: 10.1002/ar.1092110403. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Neely J. E., Ballard E. T., Britt A. L., Workman L. Characteristics of 85 pediatric tumors heterotransplanted into nude mice. Exp Cell Biol. 1983;51(4):217–227. doi: 10.1159/000163194. [DOI] [PubMed] [Google Scholar]
- SPICER S. S., WARREN L. The histochemistry of sialic acid containing mucoproteins. J Histochem Cytochem. 1960 Mar;8:135–137. doi: 10.1177/8.2.135. [DOI] [PubMed] [Google Scholar]
- Saxén L., Koskimies O., Lahti A., Miettinen H., Rapola J., Wartiovaara J. Differentiation of kidney mesenchyme in an experimental model system. Adv Morphog. 1968;7:251–293. doi: 10.1016/b978-1-4831-9954-2.50011-2. [DOI] [PubMed] [Google Scholar]
- Stoward P. J., Spicer S. S., Miller R. L. Histochemical reactivity of peanut lectin-horseradish peroxidase conjugate. J Histochem Cytochem. 1980 Sep;28(9):979–990. doi: 10.1177/28.9.7410818. [DOI] [PubMed] [Google Scholar]
- Yeger H., Baumal R., Bailey D., Pawlin G., Phillips M. J. Histochemical and immunohistochemical characterization of surgically resected and heterotransplanted Wilms' tumor. Cancer Res. 1985 May;45(5):2350–2357. [PubMed] [Google Scholar]
- Yeger H., Baumal R., Pawlin G., Phillips M. J. Relationship of histology of Wilms' tumor to growth characteristics of nude mouse heterotransplants. Cancer Res. 1985 May;45(5):2340–2349. [PubMed] [Google Scholar]