Abstract
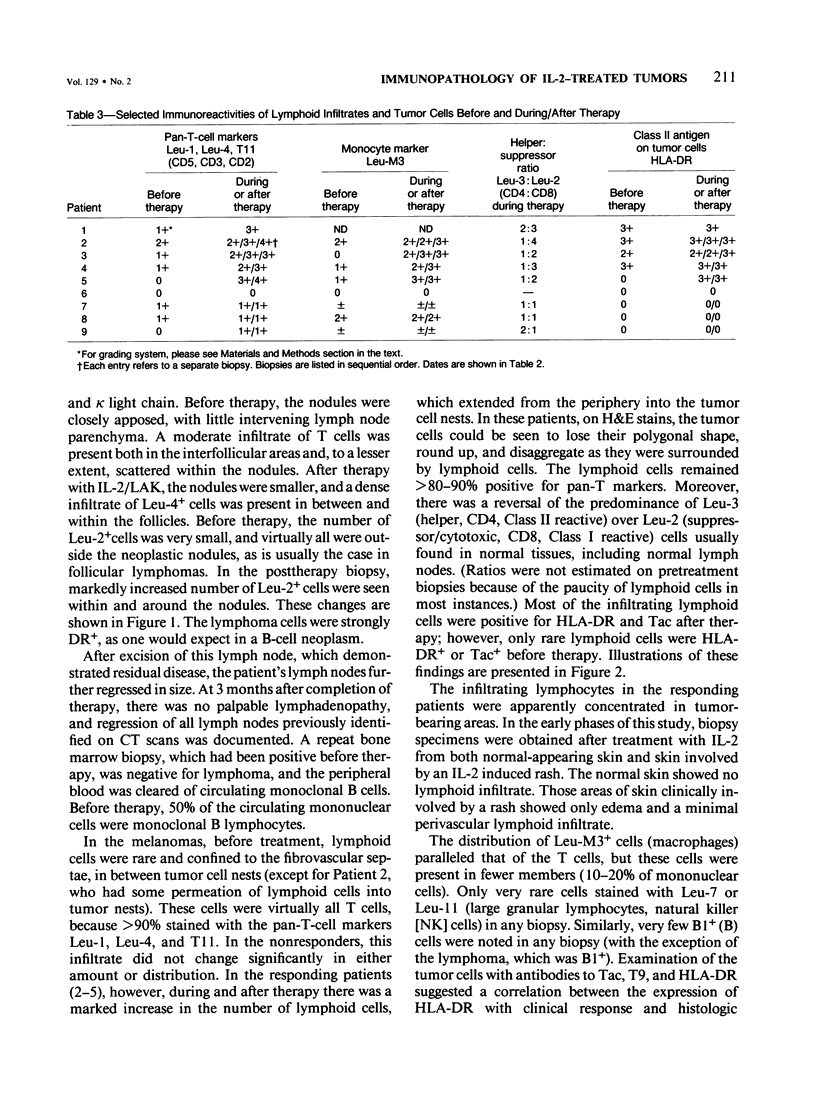
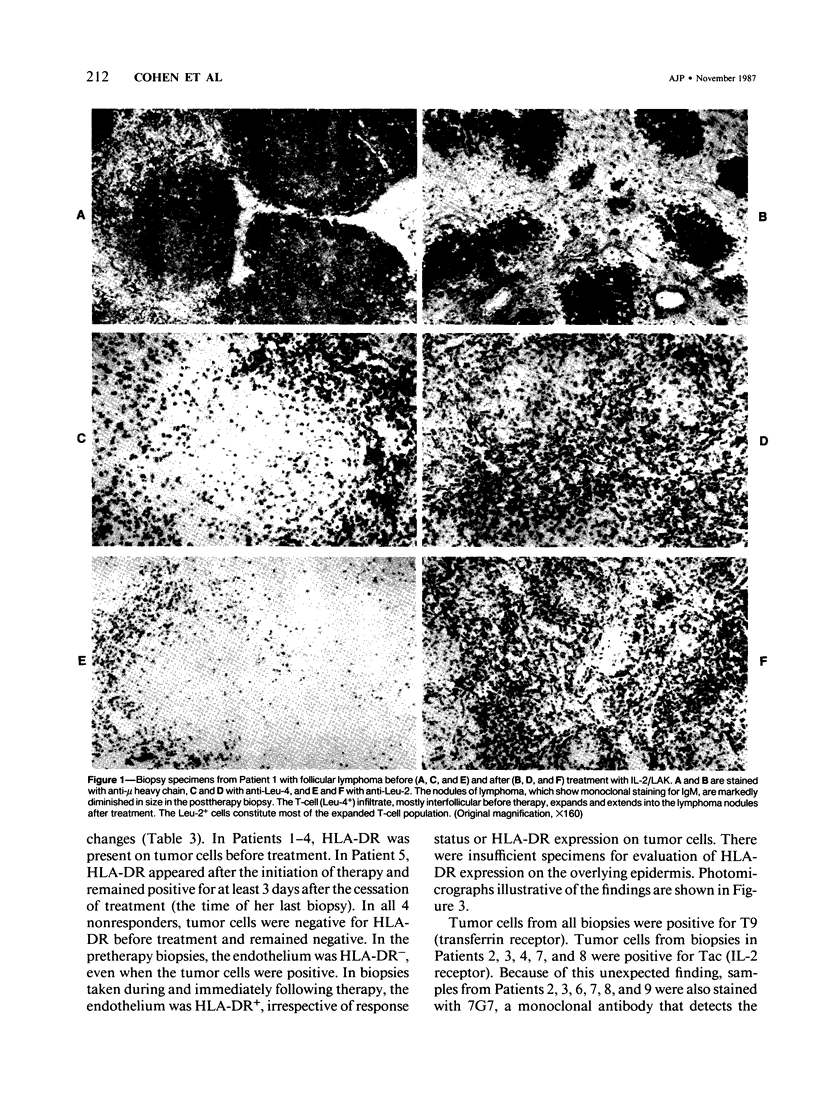
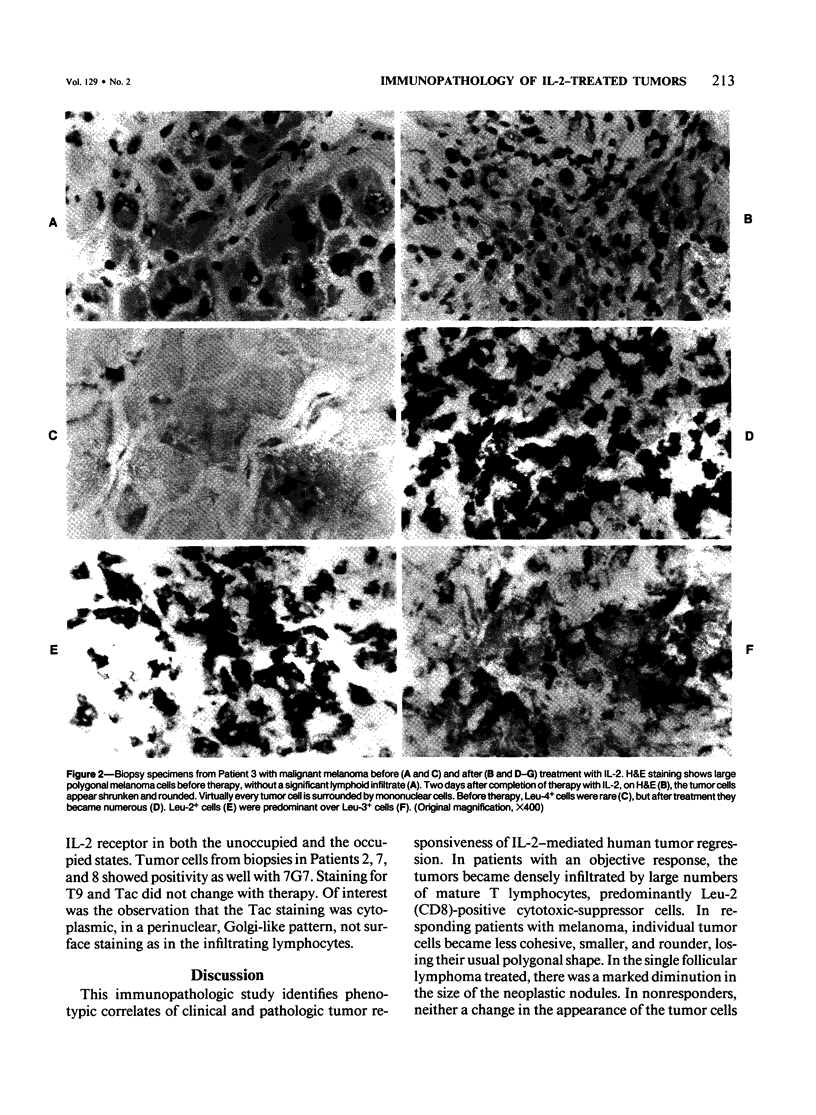
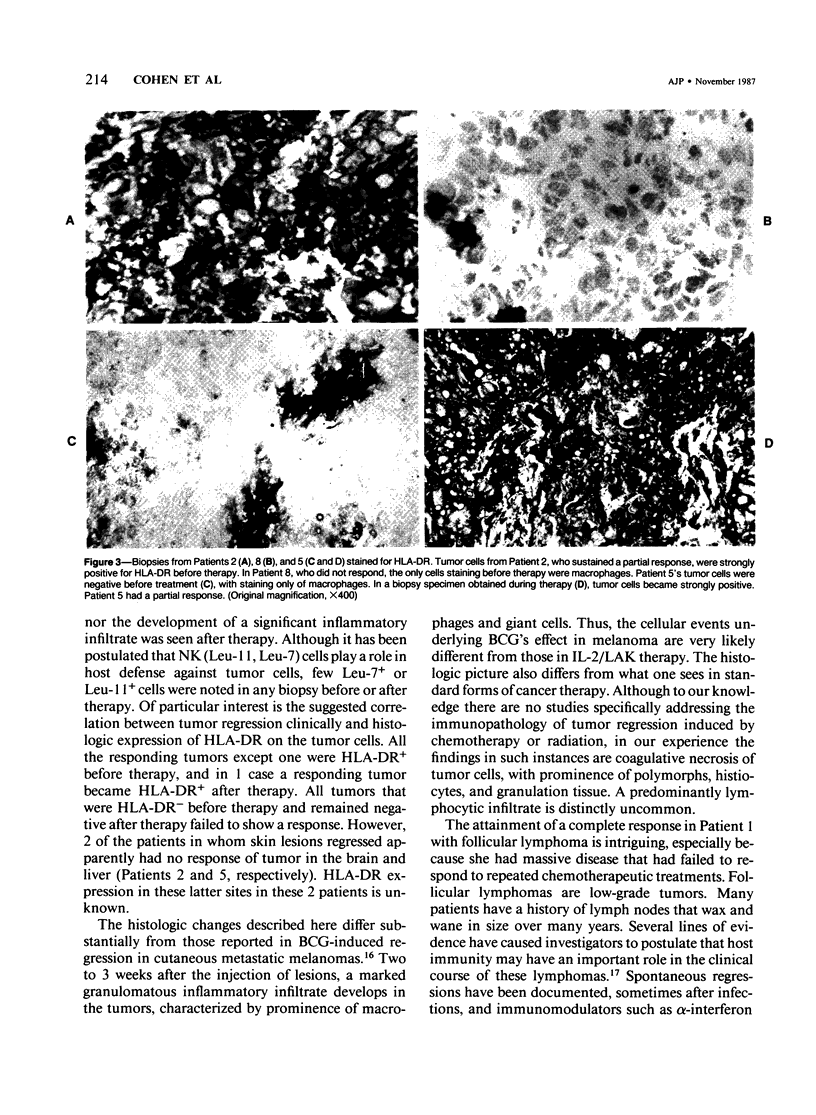
Sequential tumor biopsies from 9 patients with disseminated cancers were obtained before, during, and after treatment with interleukin-2 (IL-2) with or without the adoptive transfer of lymphokine-activated killer (LAK) cells. Infiltrating lymphoid and tumor cells were characterized in frozen sections by the use of monoclonal antibodies and the avidin-biotin complex (ABC) immunoperoxidase technique. Five patients had objective tumor regression (1 complete response of a follicular lymphoma, 4 partial responses of melanomas). Four patients (2 melanomas, 1 renal cell carcinoma, 1 breast carcinoma) were nonresponsive after treatment. After treatment, responsive tumors showed a pronounced infiltration of T cells, mainly Leu-2+ (CD8, primarily cytotoxic/suppressor) cells. Macrophages, although increased, were fewer than the T cells, and Leu-7+ or Leu-11+ (NK and K) cells were virtually absent. In nonresponders, there was no significant increase in lymphoid cells after therapy, and no differences were noted between groups before therapy. In 4 of 5 responders, tumor cells were positive for HLA-DR before therapy; and in the remaining responder, the tumor became positive during treatment. Tumor cells in all biopsy specimens from nonresponders were DR- before and after the start of therapy. It is concluded that the expression of HLA-DR by tumor cells may play a role in the response to IL-2 with or without LAK and that marked infiltration by T cells accompanies, and possibly mediates, such a response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Berinstein N., Levy R. Treatment of a murine B cell lymphoma with monoclonal antibodies and IL 2. J Immunol. 1987 Aug 1;139(3):971–976. [PubMed] [Google Scholar]
- Chang A. E., Hyatt C. L., Rosenberg S. A. Systemic administration of recombinant human interleukin-2 in mice. J Biol Response Mod. 1984 Oct;3(5):561–572. [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Dvoretsky P., Wood G. S., Levy R., Warnke R. A. T-lymphocyte subsets in follicular lymphomas compared with those in non-neoplastic lymph nodes and tonsils. Hum Pathol. 1982 Jul;13(7):618–625. doi: 10.1016/s0046-8177(82)80003-8. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Miescher S., Zocchi M. R., von Fliedner V., Moretta A. Phenotypic and functional characterization of recombinant interleukin 2 (rIL 2)-induced activated killer cells: analysis at the population and clonal levels. J Immunol. 1987 Feb 15;138(4):1297–1302. [PubMed] [Google Scholar]
- Foon K. A., Sherwin S. A., Abrams P. G., Longo D. L., Fer M. F., Stevenson H. C., Ochs J. J., Bottino G. C., Schoenberger C. S., Zeffren J. Treatment of advanced non-Hodgkin's lymphoma with recombinant leukocyte A interferon. N Engl J Med. 1984 Nov 1;311(18):1148–1152. doi: 10.1056/NEJM198411013111803. [DOI] [PubMed] [Google Scholar]
- Fossati G., Taramelli D., Balsari A., Bogdanovich G., Andreola S., Parmiani G. Primary but not metastatic human melanomas expressing DR antigens stimulate autologous lymphocytes. Int J Cancer. 1984 May 15;33(5):591–597. doi: 10.1002/ijc.2910330508. [DOI] [PubMed] [Google Scholar]
- Garcia C. F., Lowder J., Meeker T. C., Bindl J., Levy R., Warnke R. A. Differences in "host infiltrates" among lymphoma patients treated with anti-idiotype antibodies: correlation with treatment response. J Immunol. 1985 Dec;135(6):4252–4260. [PubMed] [Google Scholar]
- Guerry D., 4th, Alexander M. A., Herlyn M. F., Zehngebot L. M., Mitchell K. F., Zmijewski C. M., Lusk E. J. HLA-DR histocompatibility leukocyte antigens permit cultured human melanoma cells from early but not advanced disease to stimulate autologous lymphocytes. J Clin Invest. 1984 Jan;73(1):267–271. doi: 10.1172/JCI111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Guerry D., Koprowski H. Recombinant gamma-interferon induces changes in expression and shedding of antigens associated with normal human melanocytes, nevus cells, and primary and metastatic melanoma cells. J Immunol. 1985 Jun;134(6):4226–4230. [PubMed] [Google Scholar]
- Houghton A. N., Thomson T. M., Gross D., Oettgen H. F., Old L. J. Surface antigens of melanoma and melanocytes. Specificity of induction of Ia antigens by human gamma-interferon. J Exp Med. 1984 Jul 1;160(1):255–269. doi: 10.1084/jem.160.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Krikorian J. G., Portlock C. S., Cooney P., Rosenberg S. A. Spontaneous regression of non-Hodgkin's lymphoma: a report of nine cases. Cancer. 1980 Nov 1;46(9):2093–2099. doi: 10.1002/1097-0142(19801101)46:9<2093::aid-cncr2820460931>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Wybran J., Epstein W. The immunologic and histopathologic changes of BCG-mediated tumor regression in patients with malignant melanoma. Cancer. 1975 Mar;35(3):756–777. doi: 10.1002/1097-0142(197503)35:3<756::aid-cncr2820350331>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Lotze M. T., Rosenberg S. A. Results of clinical trials with the administration of interleukin 2 and adoptive immunotherapy with activated cells in patients with cancer. Immunobiology. 1986 Sep;172(3-5):420–437. doi: 10.1016/S0171-2985(86)80122-X. [DOI] [PubMed] [Google Scholar]
- Lynch R. Immunoregulation of malignant lymphoma. N Engl J Med. 1982 Mar 4;306(9):543–544. doi: 10.1056/NEJM198203043060912. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Rosenberg S. A. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984 Feb 1;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker T. C., Lowder J., Maloney D. G., Miller R. A., Thielemans K., Warnke R., Levy R. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985 Jun;65(6):1349–1363. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. Adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. Important Adv Oncol. 1986:55–91. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Bhan A. K., Harrist T. J., Sober A. J., Mihm M. C., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982 Dec;129(6):2808–2815. [PubMed] [Google Scholar]
- Tilden A. B., Itoh K., Balch C. M. Human lymphokine-activated killer (LAK) cells: identification of two types of effector cells. J Immunol. 1987 Feb 15;138(4):1068–1073. [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. DR (Ia-like) antigens on human melanoma cells. Serological detection and immunochemical characterization. J Exp Med. 1979 Mar 1;149(3):658–668. doi: 10.1084/jem.149.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Kunkel H. G. The human Ia system. Adv Immunol. 1979;28:221–292. [PubMed] [Google Scholar]