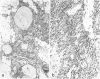
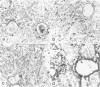
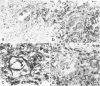
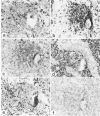
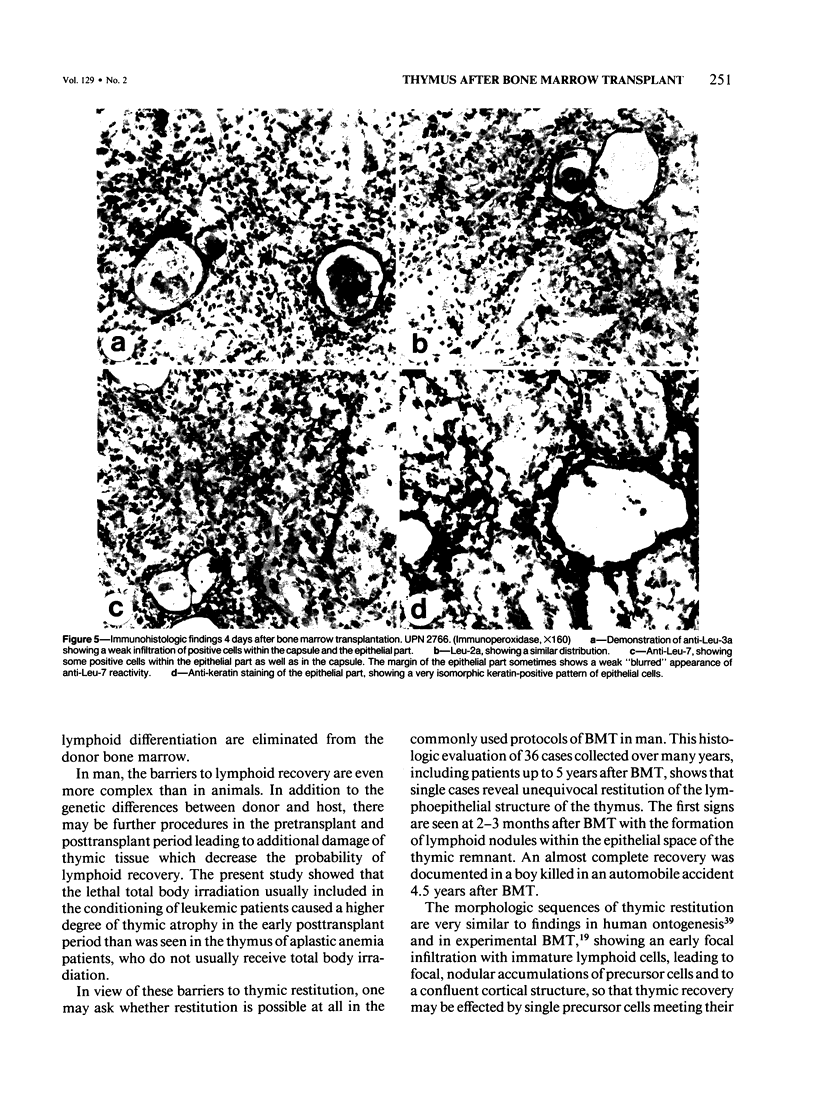
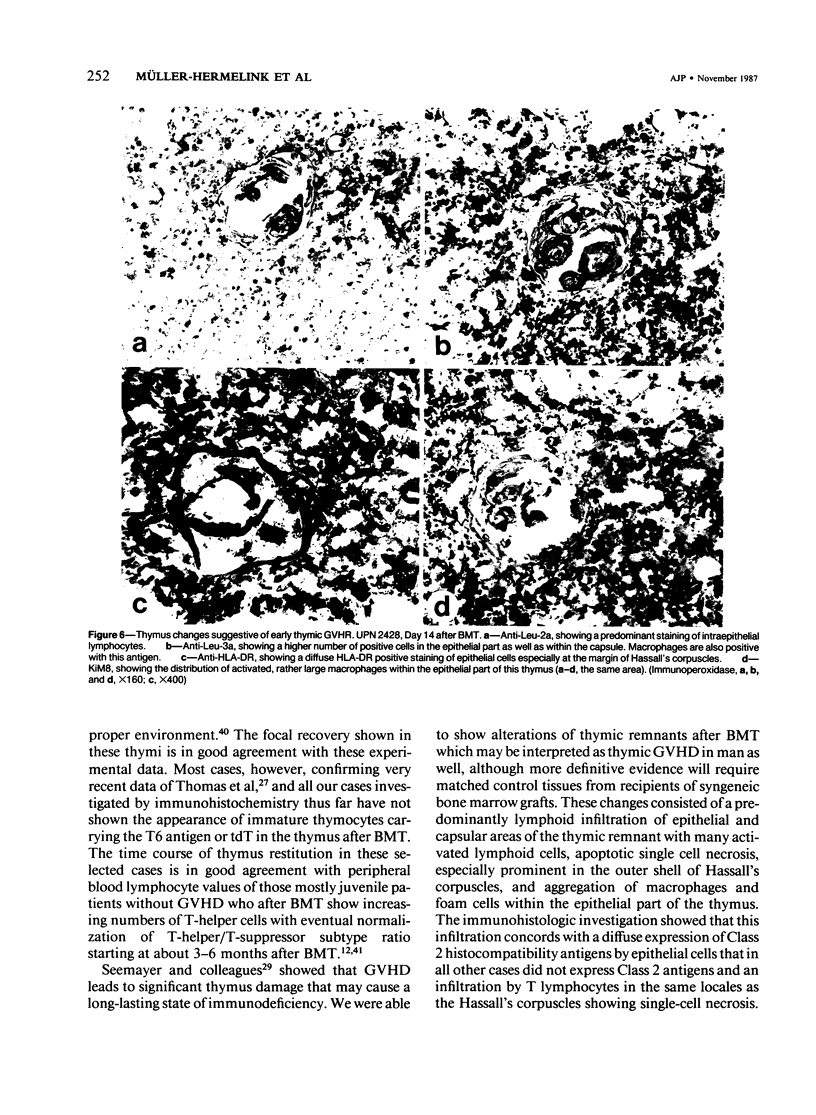
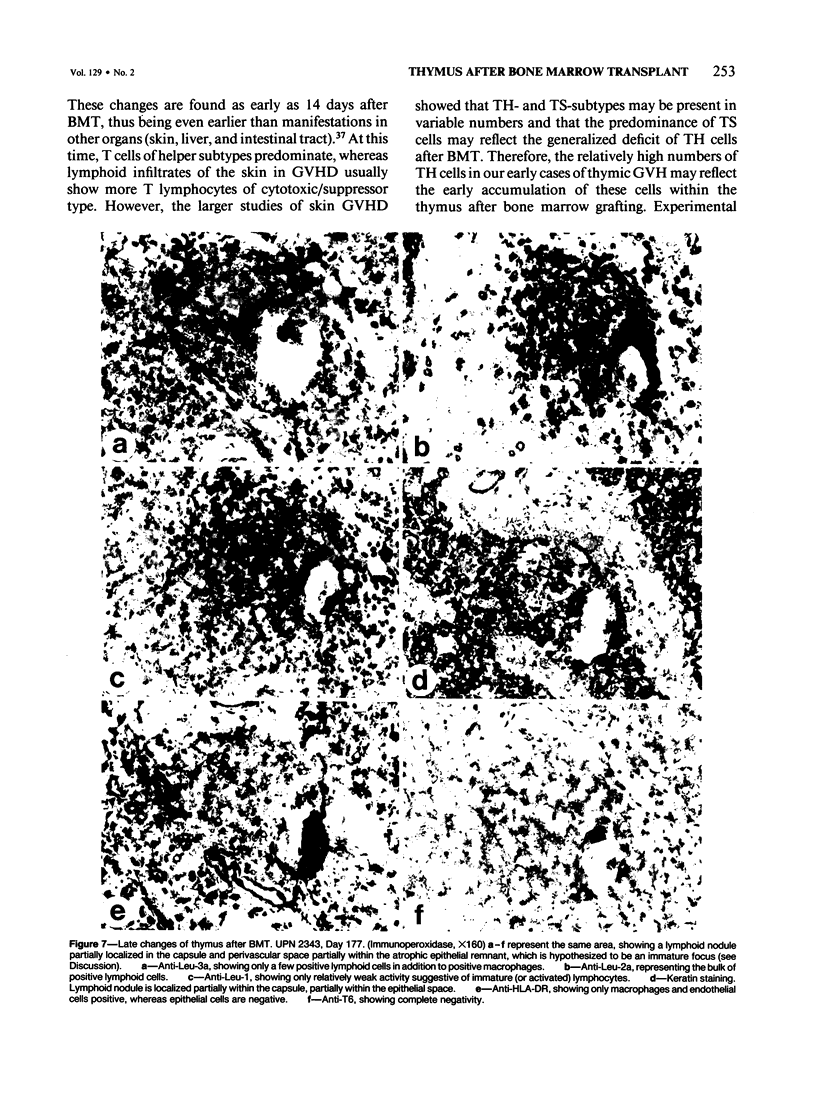
Abstract
A major hypothesis to explain the immunodeficiency associated with bone marrow transplantation states that thymic epithelial damage due to graft-versus-host disease (GVHD) abrogates or delays the recovery of normal immunologic function. This study evaluated the thymus glands of 36 human bone marrow transplant recipients dying between 4 and 1742 days after transplant using histology, histochemistry, and immunohistology. The observations lead to a model of thymic damage by irradiation, chemotherapy, and GVHD in which early injury by all three of these agents results in profound thymic atrophy followed by long-delayed restitution. Patients undergoing total body irradiation showed more severe damage to thymic cortical and medullary epithelium than did patients undergoing chemotherapy alone as preparation for transplantation. Patients with GVHD showed additional damage in the form of individual thymic epithelial cell death and showed HLA-DR surface protein expression on thymic epithelium during GVHD. Longer-term survivors showed a profoundly delayed restitution of normal thymic epithelium and delayed evidence of restored lymphopoiesis. A few patients dying late after transplant showed evidence of reconstitution of normal thymic structure or nodules of lymphopoiesis in focal areas of epithelial-cell reconstitution. Evidence of such lymphopoiesis was seen at times ranging between 90 and 1742 days after grafting. The data are consistent with a model of long-standing thymic damage caused by GVHD which is reversible after the development of tolerance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi Y. J., Barth K. C., Elfenbein G. J. T-cell phenotypic profile and colony formation during recovery from cytotoxic therapy-induced marrow aplasia. Cancer Res. 1985 Dec;45(12 Pt 1):6513–6518. [PubMed] [Google Scholar]
- Atkinson K., Incefy G. S., Storb R., Sullivan K. M., Iwata T., Dardenne M., Ochs H. D., Good R. A., Thomas E. D. Low serum thymic hormone levels in patients with chronic graft-versus-host disease. Blood. 1982 May;59(5):1073–1077. [PubMed] [Google Scholar]
- Atkinson K., Shulman H. M., Deeg H. J., Weiden P. L., Graham T. C., Thomas E. D., Storb R. Acute and chronic graft-versus-host disease in dogs given hemopoietic grafts from DLA-nonidentical littermates. Two distinct syndromes. Am J Pathol. 1982 Aug;108(2):196–205. [PMC free article] [PubMed] [Google Scholar]
- Beschorner W. E., Hutchins G. M., Elfenbein G. J., Santos G. W. The thymus in patients with allogeneic bone marrow transplants. Am J Pathol. 1978 Jul;92(1):173–186. [PMC free article] [PubMed] [Google Scholar]
- Chan W. C., Zaatari G. S., Tabei S., Bibb M., Brynes R. K. Thymoma: an immunohistochemical study. Am J Clin Pathol. 1984 Aug;82(2):160–166. doi: 10.1093/ajcp/82.2.160. [DOI] [PubMed] [Google Scholar]
- Cordier A. C., Haumont S. M. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. Am J Anat. 1980 Mar;157(3):227–263. doi: 10.1002/aja.1001570303. [DOI] [PubMed] [Google Scholar]
- Cordier A. C. Ultrastructure of the thymus in "Nude" mice. J Ultrastruct Res. 1974 Apr;47(20):26–40. doi: 10.1016/s0022-5320(74)90024-0. [DOI] [PubMed] [Google Scholar]
- Davis A. E., Jr The histopathological changes in the thymus gland in the acquired immune deficiency syndrome. Ann N Y Acad Sci. 1984;437:493–502. doi: 10.1111/j.1749-6632.1984.tb37173.x. [DOI] [PubMed] [Google Scholar]
- Favrot M., Janossy G., Tidman N., Blacklock H., Lopez E., Bofill M., Lampert I., Morgenstein G., Powles R., Prentice H. G. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983 Oct;54(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Friedrich W., O'Reilly R. J., Koziner B., Gebhard D. F., Jr, Good R. A., Evans R. L. T-lymphocyte reconstitution in recipients of bone marrow transplants with and without GVHD: imbalances of T-cell subpopulations having unique regulatory and cognitive functions. Blood. 1982 Apr;59(4):696–701. [PubMed] [Google Scholar]
- Haynes B. F., Warren R. W., Buckley R. H., McClure J. E., Goldstein A. L., Henderson F. W., Hensley L. L., Eisenbarth G. S. Demonstration of abnormalities in expression of thymic epithelial surface antigens in severe cellular immunodeficiency diseases. J Immunol. 1983 Mar;130(3):1182–1188. [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M. Pathologic appraisal of the thymus gland in acquired immunodeficiency syndrome in children. A study of four cases and a review of the literature. Arch Pathol Lab Med. 1985 Feb;109(2):142–146. [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Knott L. J., Thomas R. M., Harper P., Goldstone A. H., Davis E. G., Levinski R. J. T cell regeneration after allogeneic and autologous bone marrow transplantation. Br J Haematol. 1983 Mar;53(3):451–458. doi: 10.1111/j.1365-2141.1983.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Gülden M., Bathmann R. Restitution of the thymus in lethally irradiated mice after transplantation of syngeneic or allogeneic bone marrow. Immunobiology. 1984 Dec;167(5):462–482. doi: 10.1016/S0171-2985(84)80078-9. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Marino M., Palestro G. Pathology of thymic epithelial tumors. Curr Top Pathol. 1986;75:207–268. doi: 10.1007/978-3-642-82480-7_7. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Marino M., Palestro G., Schumacher U., Kirchner T. Immunohistological evidences of cortical and medullary differentiation in thymoma. Virchows Arch A Pathol Anat Histopathol. 1985;408(2-3):143–161. doi: 10.1007/BF00707978. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. J. Allogenic bone marrow transplantation: current status and future directions. Blood. 1983 Nov;62(5):941–964. [PubMed] [Google Scholar]
- Paulin T., Ringdén O., Lönnqvist B. Faster immunological recovery after bone marrow transplantation in patients without cytomegalovirus infection. Transplantation. 1985 Apr;39(4):377–384. doi: 10.1097/00007890-198504000-00008. [DOI] [PubMed] [Google Scholar]
- Santos G. W., Tutschka P. J., Brookmeyer R., Saral R., Beschorner W. E., Bias W. B., Braine H. G., Burns W. H., Elfenbein G. J., Kaizer H. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983 Dec 1;309(22):1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- Savino W., Dardenne M., Marche C., Trophilme D., Dupuy J. M., Pekovic D., Lapointe N., Bach J. F. Thymic epithelium in AIDS. An immunohistologic study. Am J Pathol. 1986 Feb;122(2):302–307. [PMC free article] [PubMed] [Google Scholar]
- Seddik M., Seemayer T. A., Lapp W. S. The graft-versus-host reaction and immune function. II. Recruitment of pre-T-cells in vivo by graft-versus-host-induced dysplastic thymuses following irradiation and bone marrow treatment. Transplantation. 1984 Mar;37(3):286–290. [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977 Jul;88(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Shulman H. M., Sullivan K. M., Weiden P. L., McDonald G. B., Striker G. E., Sale G. E., Hackman R., Tsoi M. S., Storb R., Thomas E. D. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- Steinmann G. G. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- Storb R., Deeg H. J., Thomas E. D., Appelbaum F. R., Buckner C. D., Cheever M. A., Clift R. A., Doney K. C., Flournoy N., Kennedy M. S. Marrow transplantation for chronic myelocytic leukemia: a controlled trial of cyclosporine versus methotrexate for prophylaxis of graft-versus-host disease. Blood. 1985 Sep;66(3):698–702. [PubMed] [Google Scholar]
- Thomas E., Storb R., Clift R. A., Fefer A., Johnson F. L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975 Apr 17;292(16):832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Sloane J. P., Imrie S. F., Ritter M. A., Schuurman H. J., Huber J. Immunohistology of the thymus in bone marrow transplant recipients. Am J Pathol. 1986 Mar;122(3):531–540. [PMC free article] [PubMed] [Google Scholar]
- Witherspoon R. P., Lum L. G., Storb R. Immunologic reconstitution after human marrow grafting. Semin Hematol. 1984 Jan;21(1):2–10. [PubMed] [Google Scholar]
- Witherspoon R. P., Matthews D., Storb R., Atkinson K., Cheever M., Deeg H. J., Doney K., Kalbfleisch J., Noel D., Prentice R. Recovery of in vivo cellular immunity after human marrow grafting. Influence of time postgrafting and acute graft-versus-host disease. Transplantation. 1984 Feb;37(2):145–150. doi: 10.1097/00007890-198402000-00006. [DOI] [PubMed] [Google Scholar]
- Witherspoon R. P., Storb R., Ochs H. D., Fluornoy N., Kopecky K. J., Sullivan K. M., Deeg J. H., Sosa R., Noel D. R., Atkinson K. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981 Aug;58(2):360–368. [PubMed] [Google Scholar]
- Witherspoon R. P., Storb R., Ochs H. D., Fluornoy N., Kopecky K. J., Sullivan K. M., Deeg J. H., Sosa R., Noel D. R., Atkinson K. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981 Aug;58(2):360–368. [PubMed] [Google Scholar]
- Zander A. R., Reuben J. M., Johnston D., Vellekoop L., Dicke K. A., Yau J. C., Hersh E. M. Immune recovery following allogeneic bone marrow transplantation. Transplantation. 1985 Aug;40(2):177–183. doi: 10.1097/00007890-198508000-00014. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B. The development of the human thymus microenvironment. Curr Top Pathol. 1986;75:1–41. doi: 10.1007/978-3-642-82480-7_1. [DOI] [PubMed] [Google Scholar]