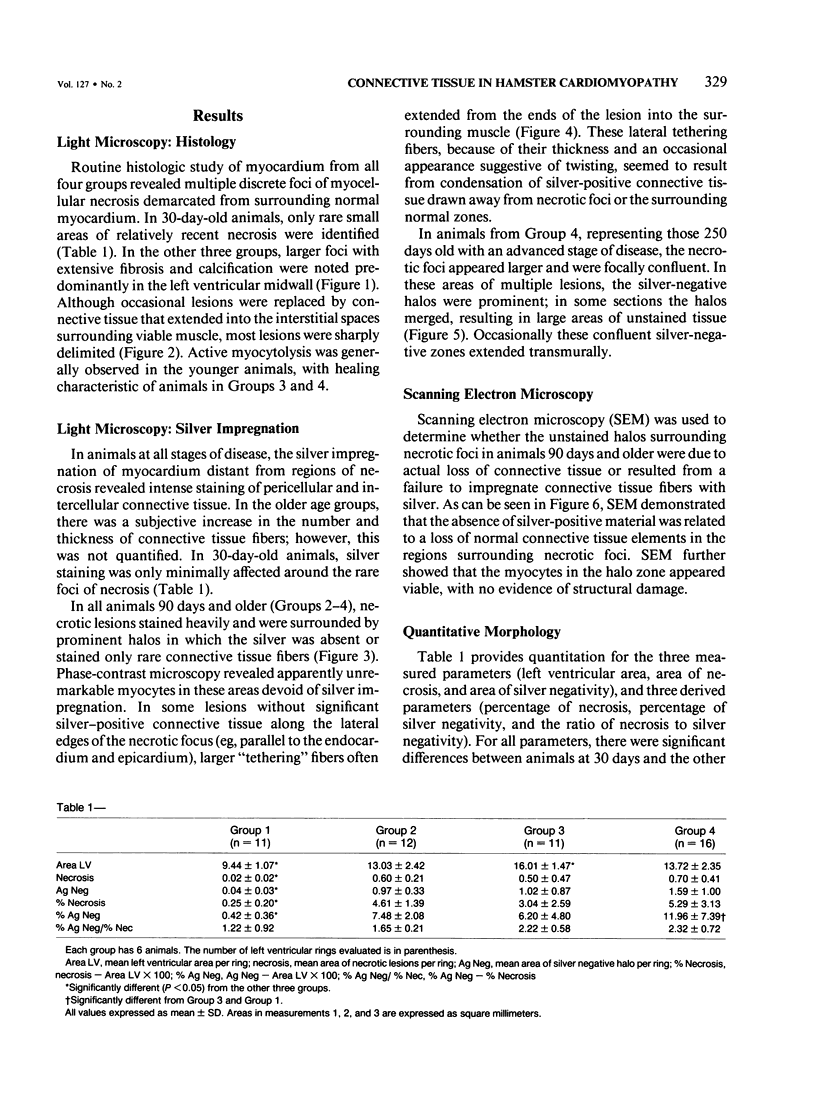
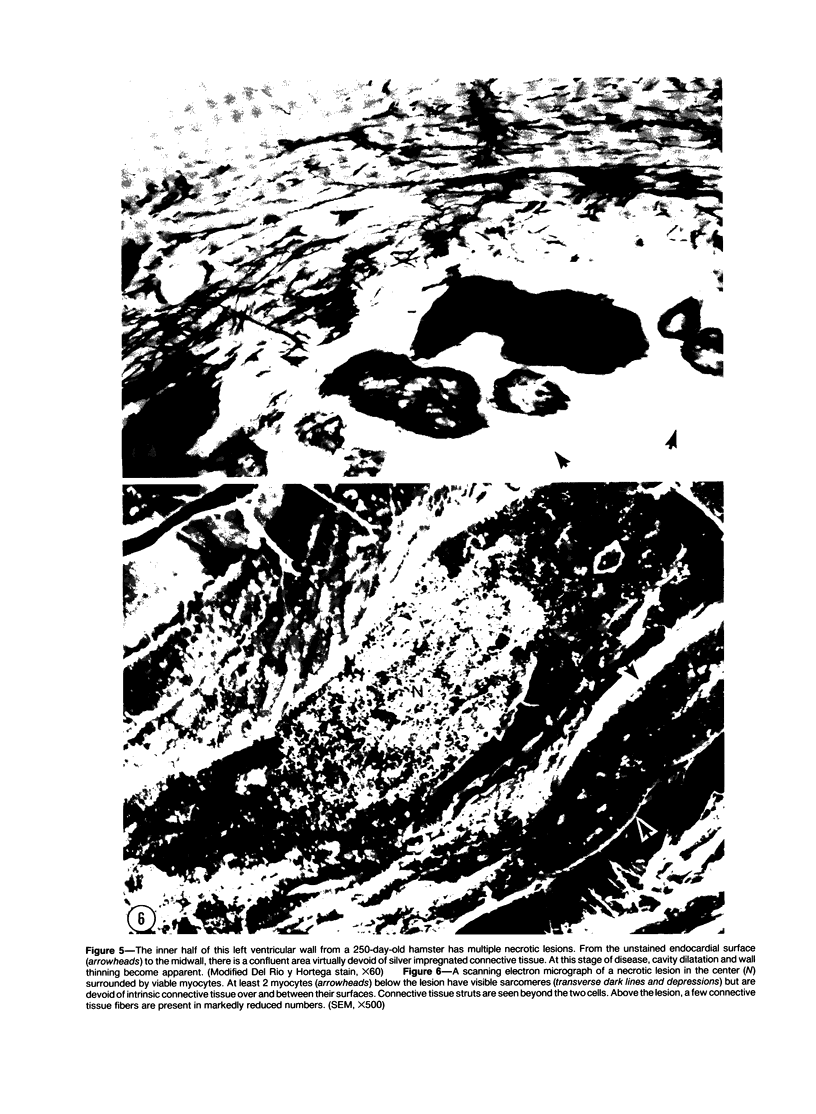
Abstract
Significant connective tissue abnormalities occurring in hearts of cardiomyopathic Syrian hamsters are reported. These abnormalities include a pronounced loss of the intrinsic connective tissue skeletal framework around foci of myocytolytic necrosis within the non-necrotic myocardium. These changes were demonstrated by a silver impregnation technique, and they were confirmed by scanning electron microscopy. Quantitation demonstrated more than a twofold increase in the area of ventricular wall affected by pathologic changes, when the connective tissue alterations were included with the myocardial necrosis. In addition, the authors also observed focal, thick "tethering" connective tissue fibers at the termini of necrotic lesions, seemingly connecting them to normal muscle. These connective tissue abnormalities may contribute to the progressive loss of ventricular function that occurs in this model of cardiomyopathy. They may permit greater wall thinning than would occur with focal necrosis alone, and they may increase focal mural stiffness in the tethered regions. Further investigation of the pathogenesis of these changes and their mechanical significance is indicated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caulfield J. B., Borg T. K. The collagen network of the heart. Lab Invest. 1979 Mar;40(3):364–372. [PubMed] [Google Scholar]
- Factor S. M., Minase T., Cho S., Dominitz R., Sonnenblick E. H. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982 Aug;66(2):342–354. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- Fedelesova M., Dhalla N. S. High energy phosphate stores in the hearts of genetically dystrophic hamsters. J Mol Cell Cardiol. 1971 Sep;3(1):93–102. doi: 10.1016/0022-2828(71)90035-6. [DOI] [PubMed] [Google Scholar]
- Gertz E. W. Cardiomyopathic Syrian hamster: a possible model of human disease. Prog Exp Tumor Res. 1972;16:242–260. doi: 10.1159/000393374. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ravens J. R. A metallic impregnation method for the demonstration of cerebral vascular patterns. Acta Neuropathol. 1968 Apr 8;10(3):183–188. doi: 10.1007/BF00687721. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Tandler B., Parland W., Turkaly J. S., Albers L. D. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982 Feb 10;257(3):1540–1548. [PubMed] [Google Scholar]
- Jasmin G., Proschek L. Hereditary polymyopathy and cardiomyopathy in the Syrian hamster. I. Progression of heart and skeletal muscle lesions in the UM-X7.1 line. Muscle Nerve. 1982 Jan;5(1):20–25. doi: 10.1002/mus.880050105. [DOI] [PubMed] [Google Scholar]
- Lochner A., Brink A. J., Van der Walt J. J. The significance of biochemical and structural changes in the development of the myocardiopathy of the Syrian hamster. J Mol Cell Cardiol. 1970 Mar;1(1):47–64. doi: 10.1016/0022-2828(70)90028-3. [DOI] [PubMed] [Google Scholar]
- Page D. L., Caulfield J. B., Kastor J. A., DeSanctis R. W., Sanders C. A. Myocardial changes associated with cardiogenic shock. N Engl J Med. 1971 Jul 15;285(3):133–137. doi: 10.1056/NEJM197107152850301. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Factor S. M. Skeletal framework of mammalian heart muscle. Arrangement of inter- and pericellular connective tissue structures. Lab Invest. 1983 Oct;49(4):482–498. [PubMed] [Google Scholar]
- Robinson T. F., Factor S. M., Sonnenblick E. H. The heart as a suction pump. Sci Am. 1986 Jun;254(6):84–91. doi: 10.1038/scientificamerican0686-84. [DOI] [PubMed] [Google Scholar]
- Robinson T. F. Lateral connections between heart muscle cells as revealed by conventional and high voltage transmission electron microscopy. Cell Tissue Res. 1980;211(3):353–359. doi: 10.1007/BF00234392. [DOI] [PubMed] [Google Scholar]
- Sato S., Ashraf M., Millard R. W., Fujiwara H., Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol. 1983 Apr;15(4):261–275. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Strobeck J. E., Factor S. M., Bhan A., Sole M., Liew C. C., Fein F., Sonnenblick E. H. Hereditary and acquired cardiomyopathies in experimental animals: mechanical, biochemical, and structural features. Ann N Y Acad Sci. 1979;317:59–88. doi: 10.1111/j.1749-6632.1979.tb56511.x. [DOI] [PubMed] [Google Scholar]
- Whitmer J. T. Energy metabolism and mechanical function in perfused hearts of Syrian hamsters with dilated or hypertrophic cardiomyopathy. J Mol Cell Cardiol. 1986 Mar;18(3):307–317. doi: 10.1016/s0022-2828(86)80413-8. [DOI] [PubMed] [Google Scholar]