Abstract
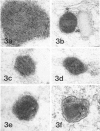
Electron-microscopic examination of five LGL clones, JT3, JTB18, CNK6, CNK7, and CNK10, expressing natural killer activity and T11 and NKH1 phenotype, showed that three of the clones, JT3, CNK6, and CNK7, had crystalline structures in their densest granules. These structures generally consisted of hexagonally packed lattices with a 6.9-nm point-to-point spacing. JTB18 and CNK10 had no structures in their granules. The attack of one clone, JT3, on two resistant target tumor cell lines, KG1 and Laz509, was also examined under three conditions. First, JT3 cells and targets were incubated together. There was little adherence, degranulation, or killing. Second, cells were incubated with anti-T11(2) and T11(3), antibodies against the E-rosette receptor/antigen complex, which activate resting T cells and enhance cytolytic activity of NK clones and CTL. JT3 cells adhered to the targets, formed zones of apposition between NK and target cell membranes, polarized, and degranulated into the space between the two cells, killing the targets. Third, cells were incubated with both anti-T11(2/3) and anti-LFA-1, an antibody that inhibits adherence. The JT3 cells did not form zones of apposition with the targets, but degranulated in discrete areas on their own surface. In all cases, discharged crystalline granules transformed to sheets of membrane and vesicles. These studies suggest that phospholipids are packed in hexagonal lattices in the granules of the resting cells and transform to bilayer structures during exocytosis. The crystalline nature of the granule may immobilize lytic molecules and protect the resting cell from lysis. Further, the vesicles may serve to transport the lytic molecules from the effector to the target cell. Anti-LFA-1 does not inhibit target recognition or exocytosis, but instead blocks membrane interactions of the effector cell with its target.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. D., Chakravarty N. Time course of anaphylactic histamine release and morphological changes in rat peritoneal mast cells. Acta Physiol Scand. 1970 Mar;78(3):410–419. doi: 10.1111/j.1748-1716.1970.tb04677.x. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Lewis R. A., Hein A., Austen K. F. Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol. 1980 May;85(2):299–312. doi: 10.1083/jcb.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Leonard R., Tardieu A., Branton D. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta. 1970;219(1):47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Dennert G. Mechanism of cell-mediated cytolysis by natural killer cells. Surv Synth Pathol Res. 1985;4(1):69–83. doi: 10.1159/000156966. [DOI] [PubMed] [Google Scholar]
- Dennert G., Podack E. R. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J Exp Med. 1983 May 1;157(5):1483–1495. doi: 10.1084/jem.157.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi C. E., Cadoni A., Zicca A., Leprini A., Ferrarini M. Large granular lymphocytes in human peripheral blood: ultrastructural and cytochemical characterization of the granules. Blood. 1982 Feb;59(2):277–283. [PubMed] [Google Scholar]
- Grossi C. E., Webb S. R., Zicca A., Lydyard P. M., Moretta L., Mingari M. C., Cooper M. D. Morphological and histochemical analyses of two human T-cell subpopulations bearing receptors for IgM or IgG. J Exp Med. 1978 May 1;147(5):1405–1417. doi: 10.1084/jem.147.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Henkart M. P., Henkart P. A. Lymphocyte mediated cytolysis as a secretory phenomenon. Adv Exp Med Biol. 1982;146:227–247. doi: 10.1007/978-1-4684-8959-0_13. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Hercend T., Meuer S., Reinherz E. L., Schlossman S. F., Ritz J. Generation of a cloned NK cell line derived from the "null cell" fraction of human peripheral blood. J Immunol. 1982 Sep;129(3):1299–1305. [PubMed] [Google Scholar]
- Junger E., Reinauer H. Liquid crystalline phases of hydrated phosphatidylethanolamine. Biochim Biophys Acta. 1969 Jul 15;183(2):304–308. doi: 10.1016/0005-2736(69)90086-8. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., GLAUERT A. M. STRUCTURE AND ASSEMBLY OF MACROMOLECULAR LIPID COMPLEXES COMPOSED OF GLOBULAR MICELLES. J Mol Biol. 1964 May;8:727–748. doi: 10.1016/s0022-2836(64)80121-2. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Schmidt R. E., Caulfield J. P., Hein A., Bartley G. T., Ritz J., Schlossman S. F., Austen K. F., Stevens R. L. Proteoglycans in cell-mediated cytotoxicity. Identification, localization, and exocytosis of a chondroitin sulfate proteoglycan from human cloned natural killer cells during target cell lysis. J Exp Med. 1985 Dec 1;162(6):1771–1787. doi: 10.1084/jem.162.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984 Jun;132(6):3197–3204. [PubMed] [Google Scholar]
- Neighbour P. A., Huberman H. S., Kress Y. Human large granular lymphocytes and natural killing ultrastructural studies of strontium-induced degranulation. Eur J Immunol. 1982 Jul;12(7):588–595. doi: 10.1002/eji.1830120711. [DOI] [PubMed] [Google Scholar]
- Payne C. M., Glasser L., Fiederlein R., Lindberg R. New ultrastructural observations: parallel tubular arrays in human T gamma lymphoid cells. J Immunol Methods. 1983 Dec 30;65(3):307–317. doi: 10.1016/0022-1759(83)90126-6. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- STOECKENIUS W. Some electron microscopical observations on liquid-crystalline phases in lipid-water systems. J Cell Biol. 1962 Feb;12:221–229. doi: 10.1083/jcb.12.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Smit J. W., Blom N. R., van Luyn M., Halie M. R. Lymphocytes with parallel tubular structures: morphologically a distinctive subpopulation. Blut. 1983 Jun;46(6):311–320. doi: 10.1007/BF00320691. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Ward J. M., Reynolds C. W. Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol. 1983 Apr;111(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Zagury D. Direct analysis of individual killer T cells: susceptibility of target cells to lysis and secretion of hydrolytic enzymes by CTL. Adv Exp Med Biol. 1982;146:149–169. doi: 10.1007/978-1-4684-8959-0_10. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Brothers M. A., Chiu F. J., Müller-Eberhard H. J. Mechanism of cytotoxicity of human large granular lymphocytes: relationship of the cytotoxic lymphocyte protein to the ninth component (C9) of human complement. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5262–5266. doi: 10.1073/pnas.83.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]