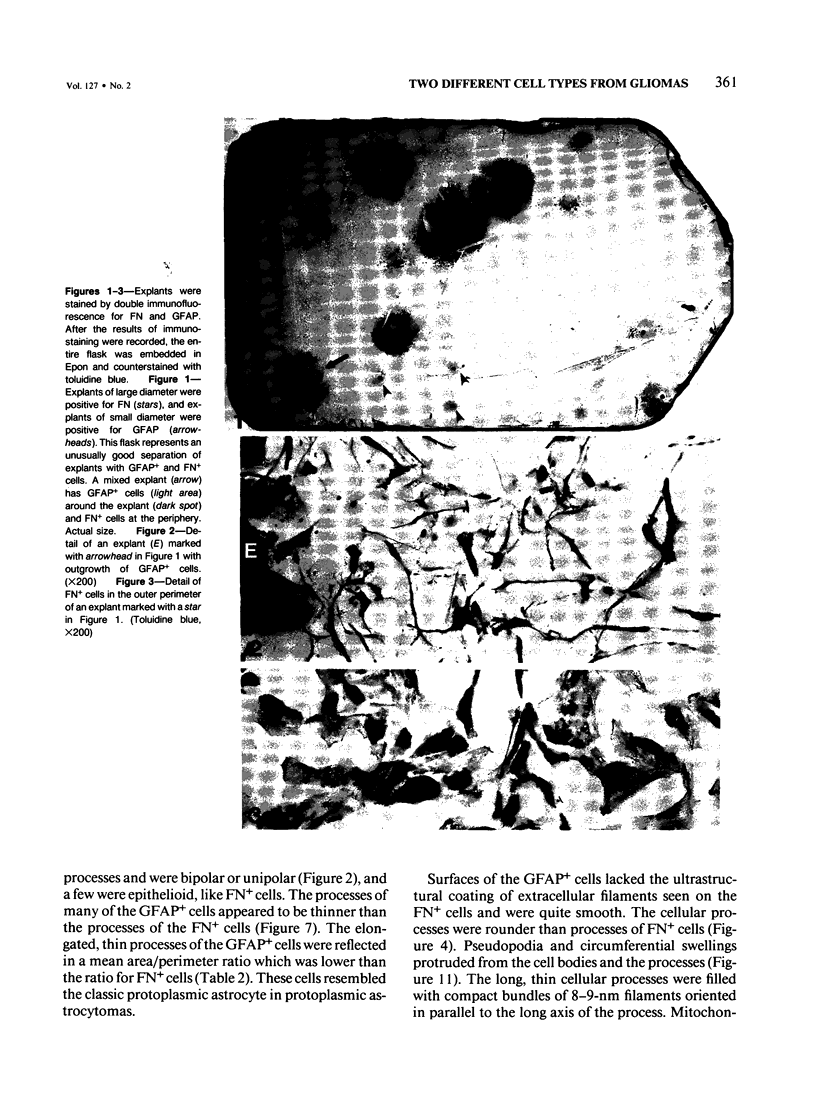
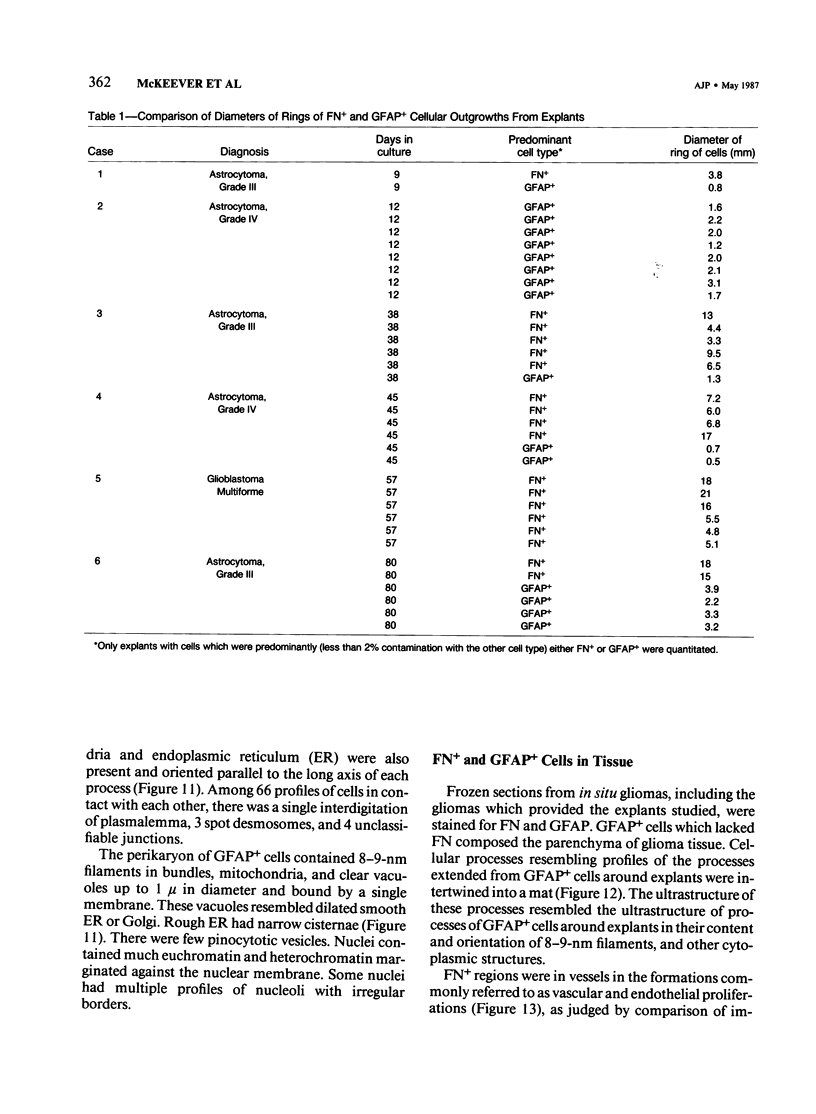
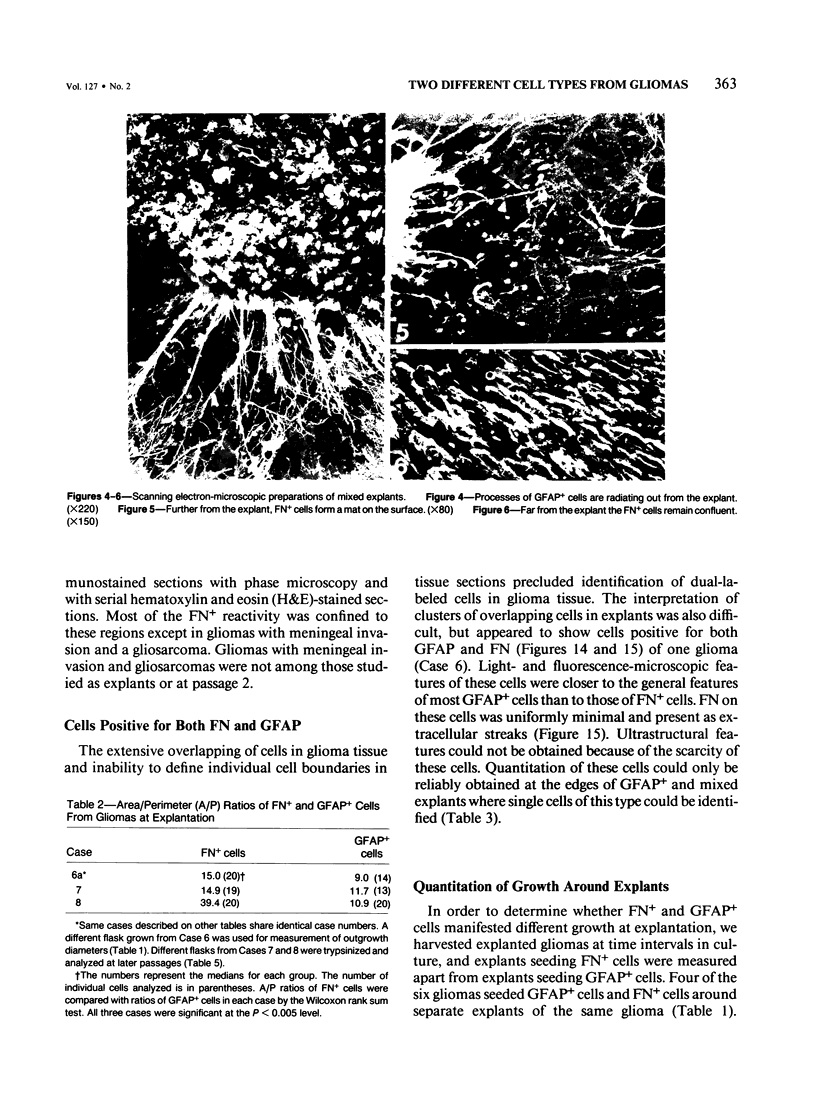
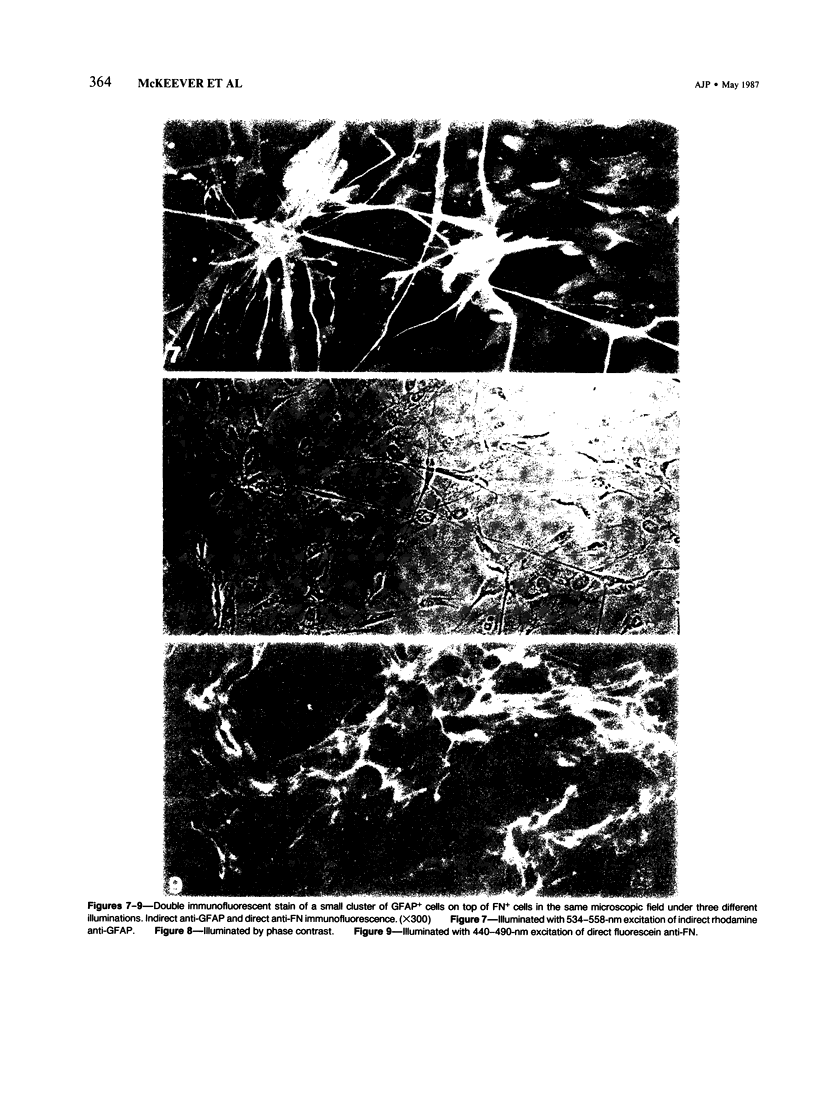
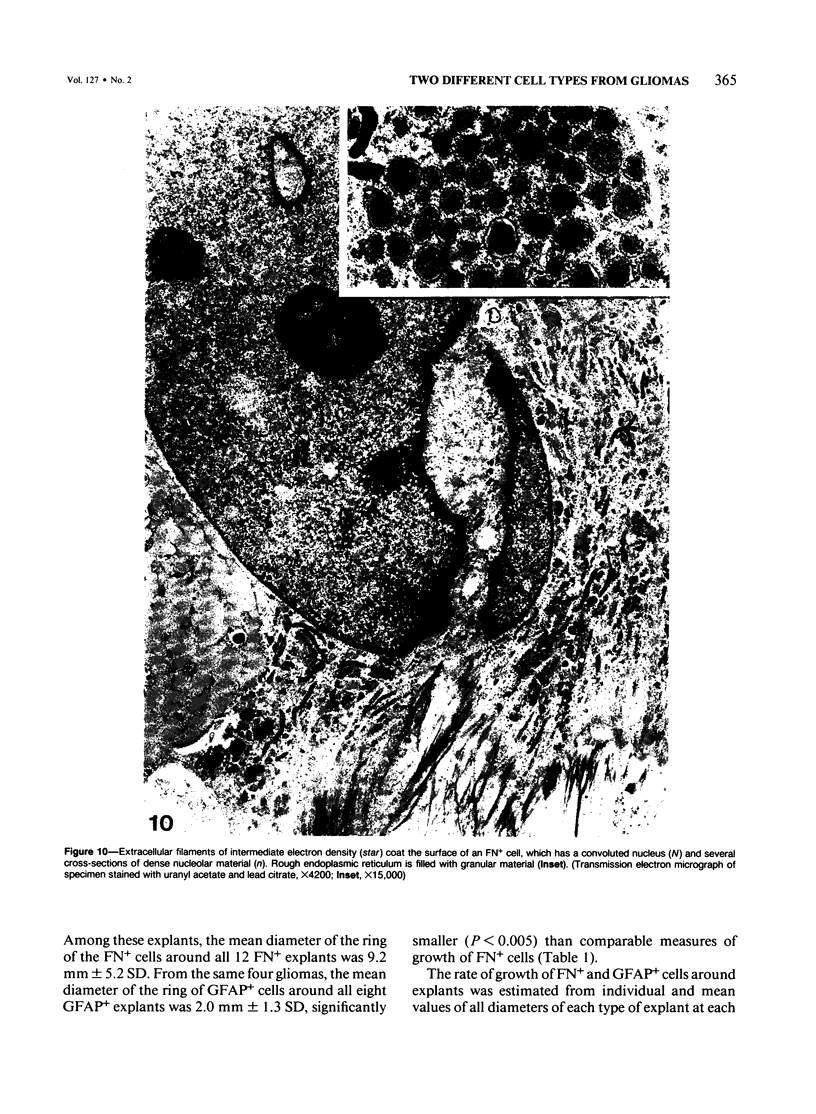
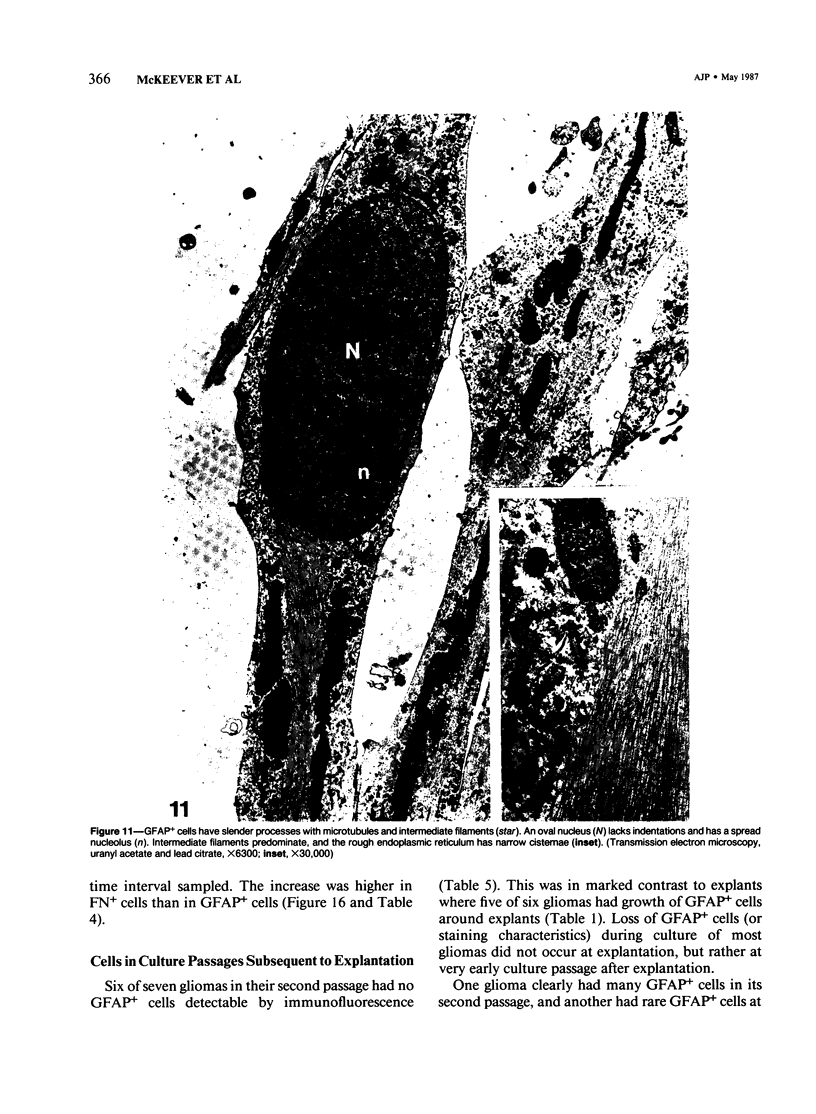
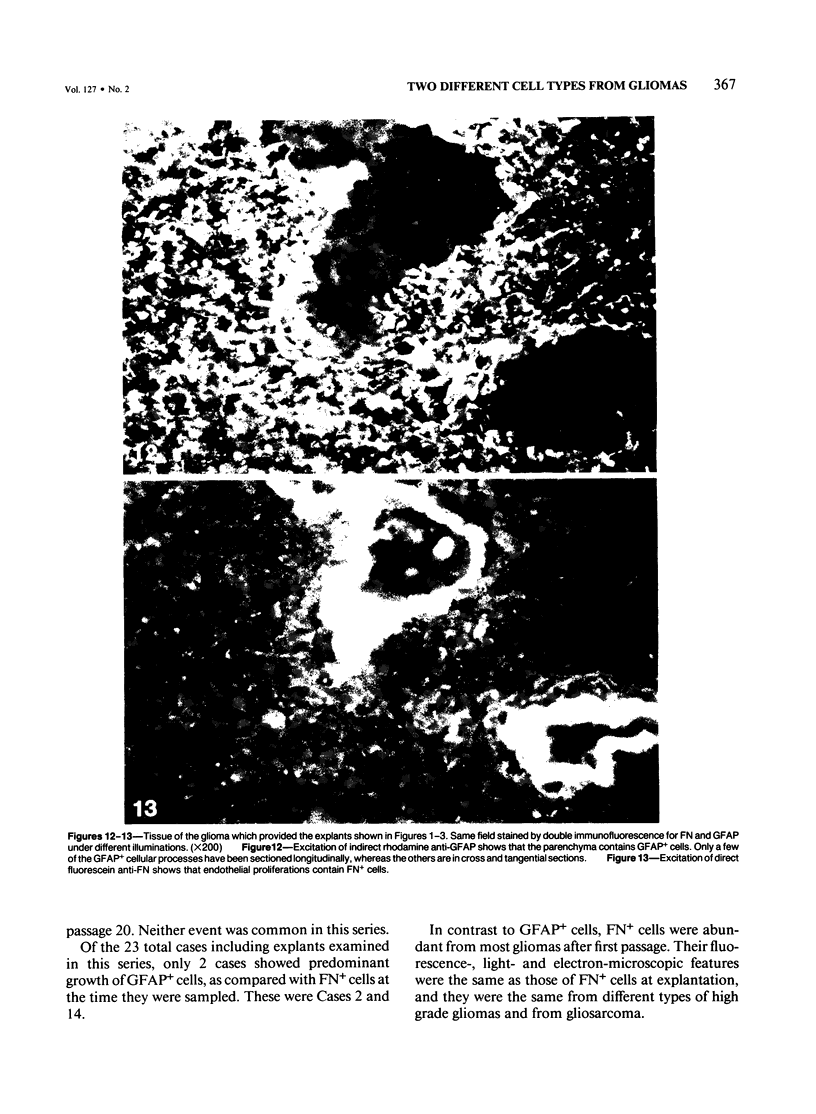
Abstract
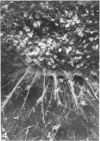
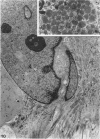
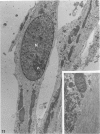
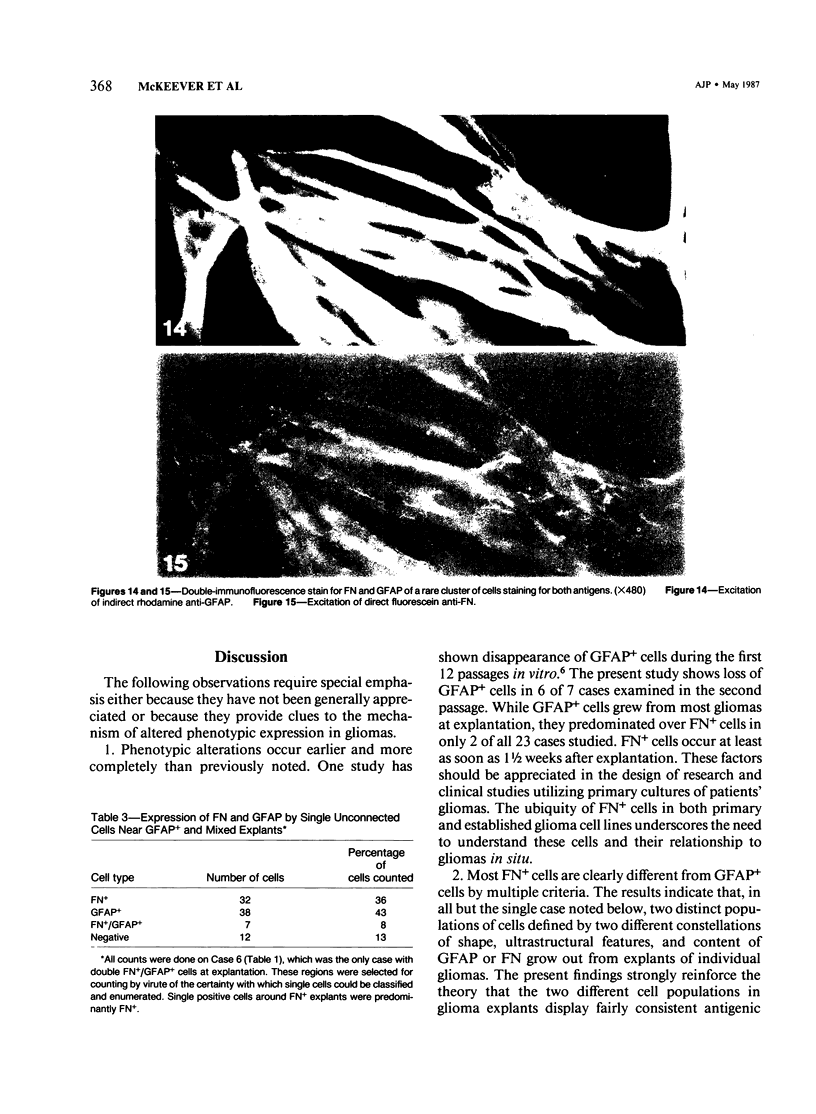
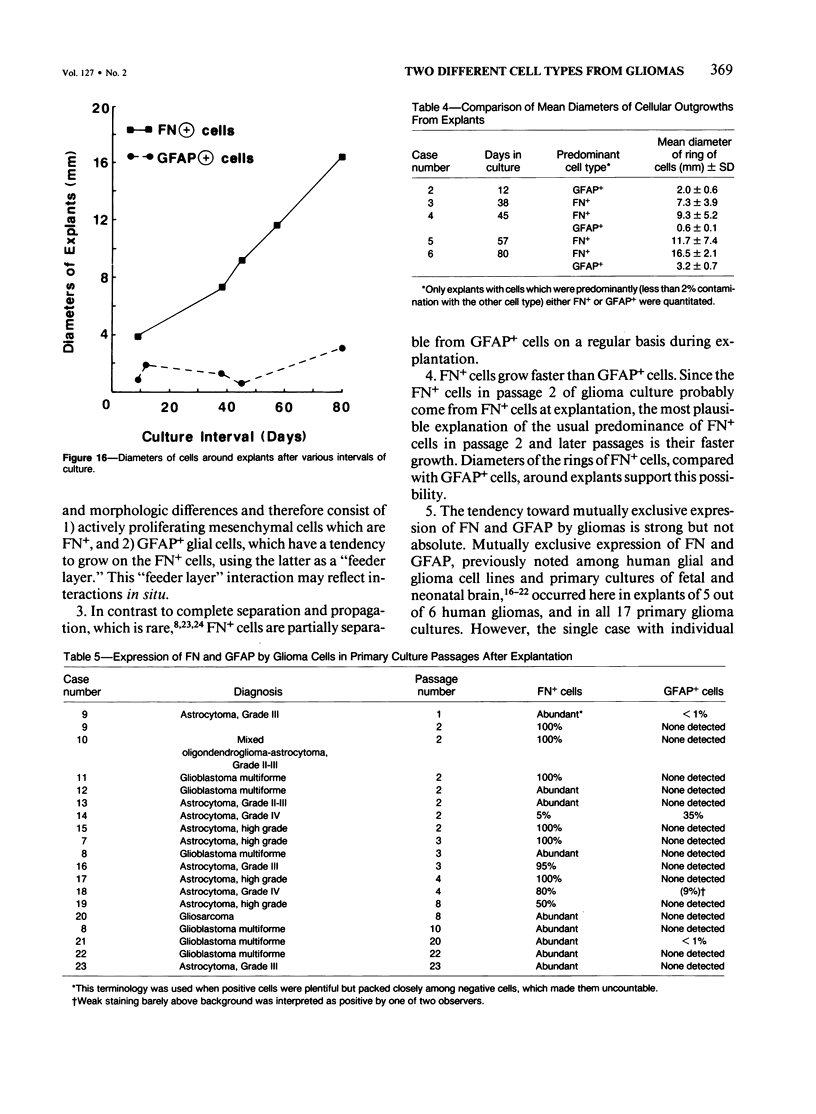
Explants derived from human gliomas have been characterized with respect to their cellular outgrowth pattern after 1-22 weeks in culture. A mat of cells which were fibronectin (FN)-positive and glial fibrillary acidic protein (GFAP)-negative (hereafter designated FN+ cells) with a polygonal, flat morphology covered the growth substrate in a swirling pattern for a mean diameter of 9.2 mm around FN+ explants. FN+ cells showed ruffled plasmalemma, dilated rough endoplasmic reticulin (RDR), and extracellular filamentous strands. Rare desmosomes were compatible with at most minor leptomeningeal components or differentiation. FN+ cells predominated in six of seven cultures at passage 2, and their features were the same from various high-grade gliomas and gliosarcoma. Around other explants, elongated or stellate cells which were GFAP+ and FN- grew in a netlike pattern with little cell-to-cell contact. These GFAP+ cells surrounded explants at a mean diameter of 2 mm, substantially less than FN+ cells (P less than 0.005), and they grew more slowly than FN+ cells around explants. GFAP+ cells had an area/perimeter ratio which was less than that of FN+ cells. GFAP+ cells contained abundant intracellular filaments, rare desmosomes, and narrow RER cisternae. In mixed explants, GFAP+ cells often grew on top of FN+ cells. Individual cells which stained for both GFAP and FN were evident only from one glioma (8% doubly positive). Cells negative for both proteins resembled FN+ cells morphologically. Frozen sections of original glioma tissue showed FN+ vessel walls and GFAP+ parenchyma. Results are evidence for very early overgrowth of a preexistent FN+ cell type distinct from the GFAP+ parenchymal cell. The features of this distinct cell type are mesenchymal and resemble the proliferating vascular elements of gliomas in situ. The tendency for GFAP+ cells to grow on top of these FN+ cells suggests a feeder layer interaction. More knowledge of the origins and interactions of these two cell types may increase our understanding of the mechanism of antigenic changes in gliomas and may provide clues to improved therapeutic approaches.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Bornstein P., Vaheri A., Sage H. Biosynthesis of an unusual collagen type by human astrocytoma cells in vitro. J Biol Chem. 1983 Feb 25;258(4):2653–2661. [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., McKeever P. E., Kornblith P. L. Glial and nonglial neoplasms evaluated on frozen section by double immunofluorescence for fibronectin and glial fibrillary acidic protein. Acta Neuropathol. 1983;59(4):283–287. doi: 10.1007/BF00691494. [DOI] [PubMed] [Google Scholar]
- Clark G. B., Henry J. M., McKeever P. E. Cerebral pilocytic astrocytoma. Cancer. 1985 Sep 1;56(5):1128–1133. doi: 10.1002/1097-0142(19850901)56:5<1128::aid-cncr2820560529>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Collins V. P., Forsby N., Brunk U. T., Ericsson J. L., Westermark B. Ultrastructural features of cultured human glia and glioma cells. Acta Pathol Microbiol Scand A. 1979 Jan;87(1):19–28. doi: 10.1111/j.1699-0463.1979.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Deck J. H., Eng L. F., Bigbee J., Woodcock S. M. The role of glial fibrillary acidic protein in the diagnosis of central nervous system tumors. Acta Neuropathol. 1978 Jun 30;42(3):183–190. doi: 10.1007/BF00690355. [DOI] [PubMed] [Google Scholar]
- Delpech B., Delpech A., Vidard M. N., Girard N., Tayot J., Clement J. C., Creissard P. Glial fibrillary acidic protein in tumours of the nervous system. Br J Cancer. 1978 Jan;37(1):33–40. doi: 10.1038/bjc.1978.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Rubinstein L. J. Contribution of immunohistochemistry to diagnostic problems of human cerebral tumors. J Histochem Cytochem. 1978 Jul;26(7):513–522. doi: 10.1177/26.7.357640. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Icard C., Liepkalns V. A., Yates A. J., Singh N. P., Stephens R. E., Hart R. W. Growth characteristics of human glioma-derived and fetal neural cells in culture. J Neuropathol Exp Neurol. 1981 Sep;40(5):512–525. doi: 10.1097/00005072-198109000-00003. [DOI] [PubMed] [Google Scholar]
- Jacque C. M., Kujas M., Poreau A., Raoul M., Collier P., Racadot J., Baumann N. GFA and S 100 protein levels as an index for malignancy in human gliomas and neurinomas. J Natl Cancer Inst. 1979 Mar;62(3):479–483. doi: 10.1093/jnci/62.3.479. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Ruoslahti E., Schold S. C., Bigner D. D. Fibronectin and glial fibrillary acidic protein expression in normal human brain and anaplastic human gliomas. Cancer Res. 1982 Jan;42(1):168–177. [PubMed] [Google Scholar]
- Kavinsky C. J., Garber B. B. Fibronectin associated with the glial component of embryonic brain cell cultures. J Supramol Struct. 1979;11(2):269–281. doi: 10.1002/jss.400110216. [DOI] [PubMed] [Google Scholar]
- Kornblith P. L. Role of tissue culture in prediction of malignancy. Clin Neurosurg. 1978;25:346–376. doi: 10.1093/neurosurgery/25.cn_suppl_1.346. [DOI] [PubMed] [Google Scholar]
- Macintyre E. H., Pontén J., Vatter A. E. The ultrastructure of human and murine astrocytes and of human fibroblasts in culture. Acta Pathol Microbiol Scand A. 1972;80(2):267–283. doi: 10.1111/j.1699-0463.1972.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Mahaley M. S., Jr, Bigner D. D., Dudka L. F., Wilds P. R., Williams D. H., Bouldin T. W., Whitaker J. N., Bynum J. M. Immunobiology of primary intracranial tumors. Part 7: Active immunization of patients with anaplastic human glioma cells: a pilot study. J Neurosurg. 1983 Aug;59(2):201–207. doi: 10.3171/jns.1983.59.2.0201. [DOI] [PubMed] [Google Scholar]
- Markesbery W. R., Lapham L. W. A correlated light and electron microscopic study of the early phase of growth in vitro of human fetal cerebellar and cerebral cortex. J Neuropathol Exp Neurol. 1974 Jan;33(1):113–127. doi: 10.1097/00005072-197401000-00009. [DOI] [PubMed] [Google Scholar]
- Maruno M., Yoshimine T., Ushio Y., Hayakawa T., Jamshid J., Bitoh S., Mogami H. [Immunohistochemical study of ethylnitrosourea-induced rat gliomas with vimentin and astroprotein (GFAP)]. No To Shinkei. 1985 Dec;37(12):1173–1179. [PubMed] [Google Scholar]
- Maunoury R. Establishment and characterization of 5 human cell lines derived from a series of 50 primary intracranial tumors. Acta Neuropathol. 1977 Jul 15;39(1):33–41. doi: 10.1007/BF00690383. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Chronwall B. M., Houff S. A., Sever J. L., Kornblith P. L., Padgett B. L., Walker D. L., London W. T. Glial and divergent cells in primate central nervous system tumors induced by JC virus isolated from human progressive multifocal leukoencephalopathy (PML). Prog Clin Biol Res. 1983;105:239–251. [PubMed] [Google Scholar]
- McKeever P. E., Fligiel S. E., Varani J., Hudson J. L., Smith D., Castle R. L., McCoy J. P. Products of cells cultured from gliomas. IV. Extracellular matrix proteins of gliomas. Int J Cancer. 1986 Jun 15;37(6):867–874. doi: 10.1002/ijc.2910370612. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Hood T. W., Varani J., Taren J. A., Beierwaltes W. H., Wahl R., Liebert M., Nguyen P. K. Products of cells cultured from gliomas. V. Cytology and morphometry of two cell types cultured from glioma. J Natl Cancer Inst. 1987 Jan;78(1):75–84. doi: 10.1093/jnci/78.1.75. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Quindlen E., Banks M. A., Williams U., Kornblith P. L., Laverson S., Greenwood M. A., Smith B. Biosynthesized products of cultured neuroglial cells: I. Selective release of proteins by cells from human astrocytomas. Neurology. 1981 Nov;31(11):1445–1452. doi: 10.1212/wnl.31.11.1445. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Wichman A., Chronwall B., Thomas C., Howard R. Sarcoma arising from a gliosarcoma. South Med J. 1984 Aug;77(8):1027–1032. doi: 10.1097/00007611-198408000-00023. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Raff M. C. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984 Feb;4(2):585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederman T., Norling B., Glimelius B., Carlsson J., Brunk U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984 Jul;44(7):3090–3097. [PubMed] [Google Scholar]
- Paetau A., Mellström K., Vaheri A., Haltia M. Distribution of a major connective tissue protein, fibronectin, in normal and neoplastic human nervous tissue. Acta Neuropathol. 1980;51(1):47–51. doi: 10.1007/BF00688849. [DOI] [PubMed] [Google Scholar]
- Paetau A., Mellström K., Westermark B., Dahl D., Haltia M., Vaheri A. Mutually exclusive expression of fibronectin and glial fibrillary acidic protein in cultured brain cells. Exp Cell Res. 1980 Oct;129(2):337–344. doi: 10.1016/0014-4827(80)90501-7. [DOI] [PubMed] [Google Scholar]
- Paetau A., Virtanen I. Cytoskeletal properties and endogenous degradation of glial fibrillary acidic protein and vimentin in cultured human glioma cells. Acta Neuropathol. 1986;69(1-2):73–80. doi: 10.1007/BF00687041. [DOI] [PubMed] [Google Scholar]
- Pontén J., Westermark B. Properties of human malignant glioma cells in vitro. Med Biol. 1978 Aug;56(4):184–193. [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman R., Westermark B., Vaheri A., Pontén J. Immunofluorescence studies on fibronectin distribution patterns during adhesion, deformation, and spreading of human glial and glioma cells. Ann N Y Acad Sci. 1978 Jun 20;312:444–449. doi: 10.1111/j.1749-6632.1978.tb16832.x. [DOI] [PubMed] [Google Scholar]
- Rutka J. T., Kleppe-Hoifodt H., Emma D. A., Giblin J. R., Dougherty D. V., McCulloch J. R., De Armond S. J., Rosenblum M. L. Characterization of normal human brain cultures. Evidence for the outgrowth of leptomeningeal cells. Lab Invest. 1986 Jul;55(1):71–85. [PubMed] [Google Scholar]
- Ryan B. R., Walters T. R. Immunologic hematologic diseases in infancy. J Med Soc N J. 1978 Jan;75(1):37–40. [PubMed] [Google Scholar]
- Schiffer D., Giordana M. T., Mauro A., Migheli A., Germano I., Giaccone G. Immunohistochemical demonstration of vimentin in human cerebral tumors. Acta Neuropathol. 1986;70(3-4):209–219. doi: 10.1007/BF00686074. [DOI] [PubMed] [Google Scholar]
- Schiffer D., Giordana M. T., Migheli A., Giaccone G., Pezzotta S., Mauro A. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 1986 May 21;374(1):110–118. doi: 10.1016/0006-8993(86)90399-9. [DOI] [PubMed] [Google Scholar]
- Schmitt H. P. Rapid anaplastic transformation in gliomas of adulthood. "Selection" in neuro-oncogenesis. Pathol Res Pract. 1983 Mar;176(2-4):313–323. doi: 10.1016/S0344-0338(83)80021-1. [DOI] [PubMed] [Google Scholar]
- Sharp G., Osborn M., Weber K. Occurrence of two different intermediate filament proteins in the same filament in situ within a human glioma cell line. An immunoelectron microscopical study. Exp Cell Res. 1982 Oct;141(2):385–395. doi: 10.1016/0014-4827(82)90227-0. [DOI] [PubMed] [Google Scholar]
- Shitara N., McKeever P. E., Whang-Peng J., Knutsen T., Smith B. H., Kornblith P. L. Flowcytometric and cytogenetic analysis of human cultured cell lines derived from high- and low-grade astrocytomas. Acta Neuropathol. 1983;60(1-2):40–48. doi: 10.1007/BF00685346. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Herman M. M., Rubinstein L. J. Electron microscopic observations on human glioblastomas and astrocytomas maintained in organ culture systems. Am J Pathol. 1973 Dec;73(3):589–606. [PMC free article] [PubMed] [Google Scholar]
- Stieg P. E., Kimelberg H. K., Mazurkiewicz J. E., Banker G. A. Distribution of glial fibrillary acidic protein and fibronectin in primary astroglial cultures from rat brain. Brain Res. 1980 Oct 20;199(2):493–500. doi: 10.1016/0006-8993(80)90709-x. [DOI] [PubMed] [Google Scholar]
- Tölle H. G., Weber K., Osborn M. Microinjection of monoclonal antibodies to vimentin, desmin, and GFA in cells which contain more than one IF type. Exp Cell Res. 1986 Feb;162(2):462–474. doi: 10.1016/0014-4827(86)90350-2. [DOI] [PubMed] [Google Scholar]
- Uyeda C. T., Eng L. F., Bignami A. Immunological study of the glial fibrillary acidic protein. Brain Res. 1972 Feb 11;37(1):81–89. doi: 10.1016/0006-8993(72)90347-2. [DOI] [PubMed] [Google Scholar]
- Vidard M. N., Girard N., Chauzy C., Delpech B., Delpech A., Maunoury R., Laumonier R. Disparition de la protéine gliofibrillaire (GFA) au cours de la culture de cellules de glioblastomes. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jun 19;286(24):1837–1840. [PubMed] [Google Scholar]
- Westermark B., Larsson E., Brunk U., Lubitz W., Mark J. Establishment of attached and non-attached cell lines from an uncommon human glioma. Int J Cancer. 1981 Sep 15;28(3):341–351. doi: 10.1002/ijc.2910280314. [DOI] [PubMed] [Google Scholar]
- Westermark B., Nister M., Heldin C. H. Growth factors and oncogenes in human malignant glioma. Neurol Clin. 1985 Nov;3(4):785–799. [PubMed] [Google Scholar]
- Yung W. K., Luna M., Borit A. Vimentin and glial fibrillary acidic protein in human brain tumors. J Neurooncol. 1985;3(1):35–38. doi: 10.1007/BF00165169. [DOI] [PubMed] [Google Scholar]