Abstract
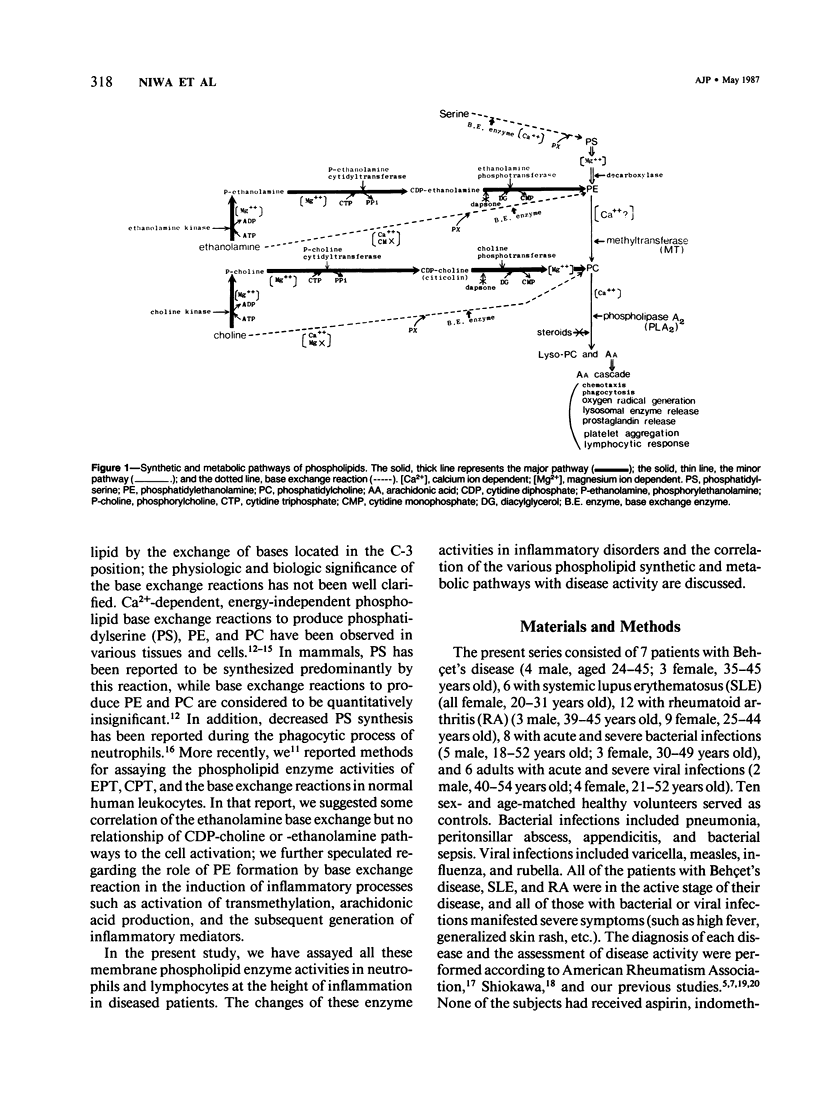
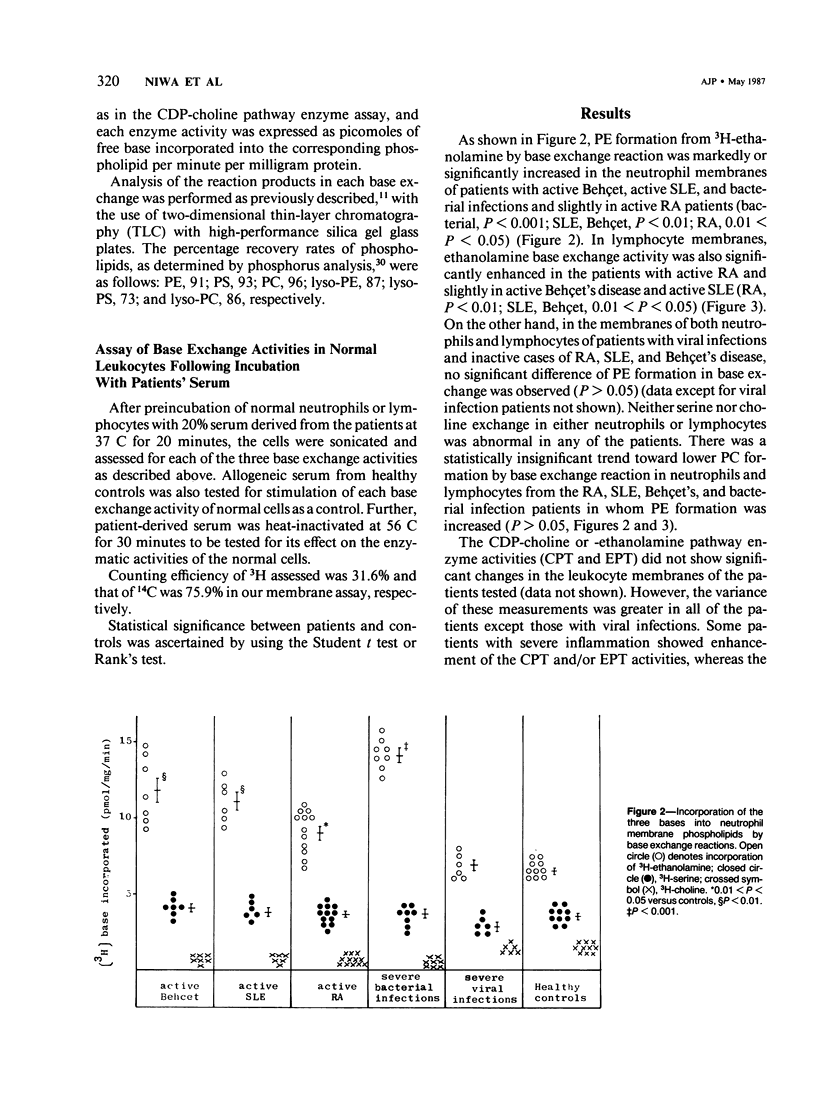
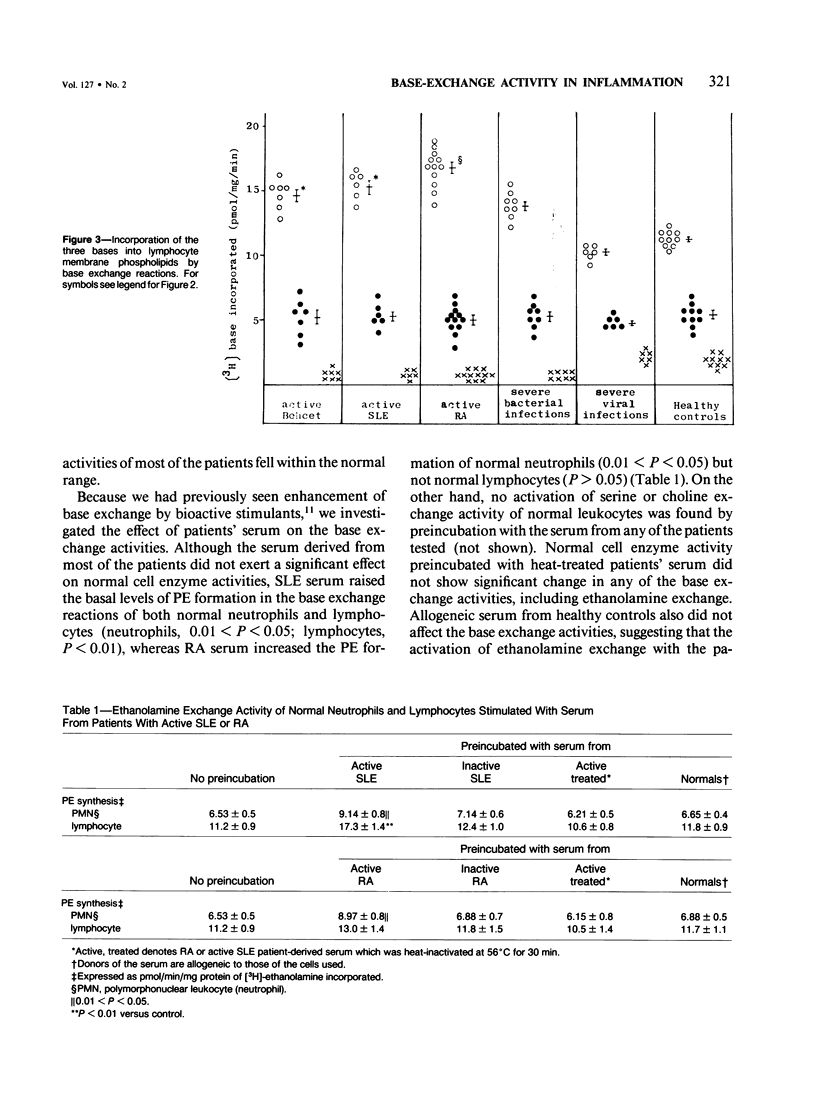
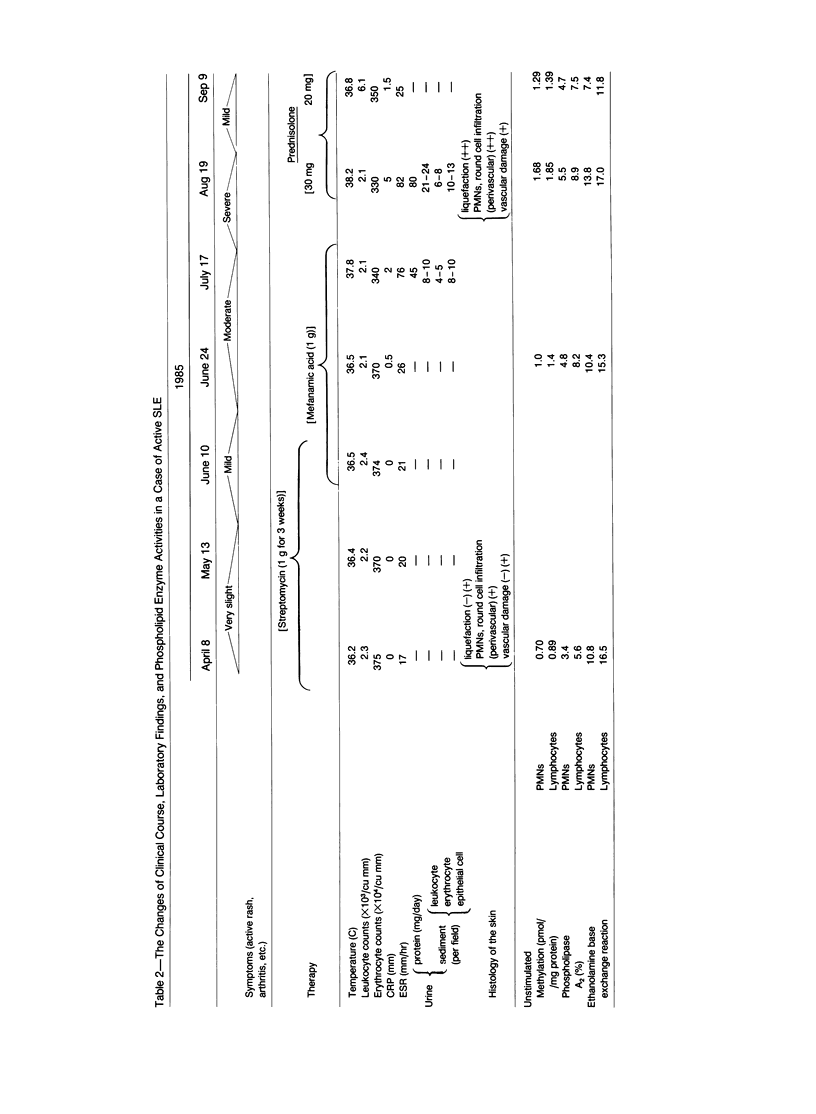
Phospholipid base exchange and cholinephosphotransferase (CPT) and ethanolaminephosphotransferase (EPT) activities were assessed in the membranes of neutrophils or lymphocytes from patients with various inflammatory disorders. Ethanolamine exchange activity was significantly enhanced in both neutrophils and lymphocytes from patients with active Behçet's disease, active systemic lupus erythematosus (SLE), and severe bacterial infections and slightly enhanced in those from patients with active rheumatoid arthritis (RA), compared with healthy controls. No abnormal findings were found in CPT, EPT, or serine or choline base exchange activities in the leukocytes from any of the diseased groups tested or in the ethanolamine exchange activity of patients with severe viral infections and inactive SLE, RA, and Behçet's disease. The authors have recently demonstrated the enhancement of transmethylation and phospholipase A2 activity in human leukocyte membranes at the height of inflammatory disease states, as well as the activation of leukocyte ethanolamine exchange by bioactive stimulants. These data postulate that phosphatidylethanolamine synthesis by the base exchange reaction may be the precursor of transmethylation and its subsequent activation of phospholipase A2, leading to the induction of arachidonic acid cascade.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Abramson S. B., Given W. P., Edelson H. S., Weissmann G. Neutrophil aggregation induced by sera from patients with active systemic lupus erythematosus. Arthritis Rheum. 1983 May;26(5):630–636. doi: 10.1002/art.1780260509. [DOI] [PubMed] [Google Scholar]
- Aksamit R. R., Backlund P. S., Jr, Cantoni G. L. Chemotaxis and the synthesis of specific proteins are inhibited by 3-deazaadenosine and other adenosine analogs in a mouse macrophage cell line. J Biol Chem. 1983 Jan 10;258(1):20–23. [PubMed] [Google Scholar]
- BEVANS M., NADELL J., DEMARTINI F., RAGAN C. The systemic lesions of malignant rheumatoid arthritis. Am J Med. 1954 Feb;16(2):197–211. doi: 10.1016/0002-9343(54)90335-6. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S. The Ca 2+ -dependent biosynthesis of lecithin, phosphatidyl-ethanolamine and phosphatidylserine in rat liver subcellular particles. Biochim Biophys Acta. 1973 Mar 8;296(3):549–562. [PubMed] [Google Scholar]
- Chien M. M., Ashman R. F. Phospholipid transmethylation in human mononuclear cells is not influenced by mitogens. Mol Immunol. 1984 Jul;21(7):621–625. doi: 10.1016/0161-5890(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Morita Y., Hirata F., Axelrod J., Siraganian R. P. Phospholipid methylation affects immunoglobulin E-mediated histamine and arachidonic acid release in rat leukemia basophils. Biochem Biophys Res Commun. 1980 Mar 13;93(1):42–49. doi: 10.1016/s0006-291x(80)80243-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro I., Mato J. M., Vasanthakumar G., Wiesmann W. P., Schiffmann E., Chiang P. K. Paradoxical effects of adenosine on neutrophil chemotaxis. J Biol Chem. 1983 Apr 10;258(7):4345–4349. [PubMed] [Google Scholar]
- Gil M. G., Alonso F., Sánchez-Crespo M., Mato J. M. Inhibition of phospholipid methyltransferase during zymosan induced secretion of platelet-activating factor in human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1981 Aug 14;101(3):740–748. doi: 10.1016/0006-291x(81)91813-1. [DOI] [PubMed] [Google Scholar]
- HUBSCHER G. Metabolism of phospholipids. VI. The effect of metal ions on the incorporation of L-serine into phosphatidylserine. Biochim Biophys Acta. 1962 Mar 12;57:555–561. doi: 10.1016/0006-3002(62)91163-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Ziff M., Hurd E. R. Increased endothelial cell adherence, aggregation, and superoxide generation by neutrophils incubated in systemic lupus erythematosus and Felty's syndrome sera. Arthritis Rheum. 1982 Dec;25(12):1409–1418. doi: 10.1002/art.1780251204. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub B. J. Differential utilization of 1-palmitoyl and 1-stearoyl homologues of various unsaturated 1,2-diacyl-sn-glycerols for phosphatidylcholine and phosphatidylethanolamine synthesis in rat liver microsomes. J Biol Chem. 1978 Feb 10;253(3):691–696. [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Solubilization and purification of rat liver microsomal 1,2-diacylglycerol: CDP-choline cholinephosphotransferase and 1,2-diacylglycerol: CDP-ethanolamine ethanolaminephosphotransferase. Eur J Biochem. 1976 Jun 15;66(1):201–210. doi: 10.1111/j.1432-1033.1976.tb10440.x. [DOI] [PubMed] [Google Scholar]
- Matsumura N., Mizushima Y. Leucocyte movement and colchicine treatment in Behcet's disease. Lancet. 1975 Oct 25;2(7939):813–813. doi: 10.1016/s0140-6736(75)80031-6. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Early increases in phospholipid methylation are not necessary for the mitogenic stimulation of lymphocytes. J Biol Chem. 1982 Jul 25;257(14):8183–8189. [PubMed] [Google Scholar]
- Morikawa S., Taniguchi S., Mori K., Kumada K., Fujiwara M., Fujiwara M. Effects of calmodulin antagonists and calmodulin on phospholipid base-exchange activities in rabbit platelets. Thromb Res. 1985 Jan 15;37(2):267–278. doi: 10.1016/0049-3848(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Kanoh T. Immune deficiency states and immune imbalance in systemic lupus erythematosus and other autoimmune diseases. Clin Immunol Immunopathol. 1979 Mar;12(3):289–300. doi: 10.1016/0090-1229(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Miyake S., Sakane T., Shingu M., Yokoyama M. Auto-oxidative damage in Behçet's disease--endothelial cell damage following the elevated oxygen radicals generated by stimulated neutrophils. Clin Exp Immunol. 1982 Jul;49(1):247–255. [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Shingu M., Miyachi Y. Role of stimulated neutrophils from patients with systemic lupus erythematosus in tissue injury, with special reference to serum factors and increased active oxygen species generated by neutrophils. Inflammation. 1985 Jun;9(2):163–172. doi: 10.1007/BF00917588. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Taniguchi S. Phospholipid transmethylation in the membrane of human neutrophils and lymphocytes. Arch Biochem Biophys. 1984 Oct;234(1):7–14. doi: 10.1016/0003-9861(84)90318-7. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Yamamoto S., Kano T., Taniguchi S. Methyltransferase and phospholipase A2 activity in membranes of neutrophils and lymphocytes from patients with bacterial and viral infections. Inflammation. 1985 Mar;9(1):53–65. doi: 10.1007/BF00915412. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Taniguchi S. Phospholipid base exchange in human leukocyte membranes: quantitation and correlation with other phospholipid biosynthetic pathways. Arch Biochem Biophys. 1986 Nov 1;250(2):345–357. doi: 10.1016/0003-9861(86)90736-8. [DOI] [PubMed] [Google Scholar]
- Ohara M., Shirado M., Miyata M., Sakuma H., Nanamiya M., Yoshida N., Yoshida H., Kasukawa R. Natural and antibody-dependent cellular cytotoxicity of polymorphonuclear leukocytes. Tohoku J Exp Med. 1983 May;140(1):59–66. doi: 10.1620/tjem.140.59. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Inhibition of superoxide anion production in macrophages by anti-inflammatory drugs. Biochem Pharmacol. 1976 Jul 1;25(13):1473–1480. doi: 10.1016/0006-2952(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Parola A. H., Kaplan J. H., Lockwood S. H., Uzgiris E. E. Activation of human lymphocytes by concanavalin A or purified protein derivative results in no alteration of fluorescence polarization of lipid probes although the electrophoretic mobility of the cells is changed. Biochim Biophys Acta. 1981 Dec 21;649(3):616–624. doi: 10.1016/0005-2736(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3928–3932. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcellati G., Arienti G., Pirotta M., Giorgini D. Base-exchange reactions for the synthesis of phospholipids in nervous tissue: the incorporation of serine and ethanolamine into the phospholipids of isolated brain microsomes. J Neurochem. 1971 Aug;18(8):1395–1417. doi: 10.1111/j.1471-4159.1971.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Sakane T., Ueda Y., Suzuki N., Niwa Y., Hoshino T., Tsunematsu T. OKT4+ and OKT8+ T lymphocytes produce soluble factors that can modulate growth and differentiation of human B cells. Clin Exp Immunol. 1985 Oct;62(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Katsuta Y., Oshima Y. Immunological studies on Behçet's syndrome. Ann Rheum Dis. 1965 Sep;24(5):494–500. doi: 10.1136/ard.24.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Hirata F., Axelrod J. Phospholipid methylation unmasks cryptic beta-adrenergic receptors in rat reticulocytes. Science. 1979 Jun 15;204(4398):1205–1207. doi: 10.1126/science.221977. [DOI] [PubMed] [Google Scholar]
- Tou J. S. Decreased incorporation of L-[U-14C]serine into phosphatidylserine by polymorphonuclear leukocytes during phagocytosis. Biochim Biophys Acta. 1979 Dec 18;575(3):384–388. doi: 10.1016/0005-2760(79)90107-3. [DOI] [PubMed] [Google Scholar]
- Vance D. E., de Kruijff B. The possible functional significance of phosphatidylethanolamine methylation. Nature. 1980 Nov 20;288(5788):277–279. doi: 10.1038/288277a0. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vial H. J., Thuet M. J., Philippot J. R. Cholinephosphotransferase and ethanolaminephosphotransferase activities in Plasmodium knowlesi-infected erythrocytes. Their use as parasite-specific markers. Biochim Biophys Acta. 1984 Sep 12;795(2):372–383. doi: 10.1016/0005-2760(84)90088-2. [DOI] [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- Weinhold P. A., Rethy V. B. The separation, purification, and characterization of ethanolamine kinase and choline kinase from rat liver. Biochemistry. 1974 Dec 3;13(25):5135–5141. doi: 10.1021/bi00722a013. [DOI] [PubMed] [Google Scholar]


