Abstract
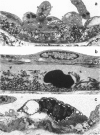
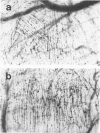
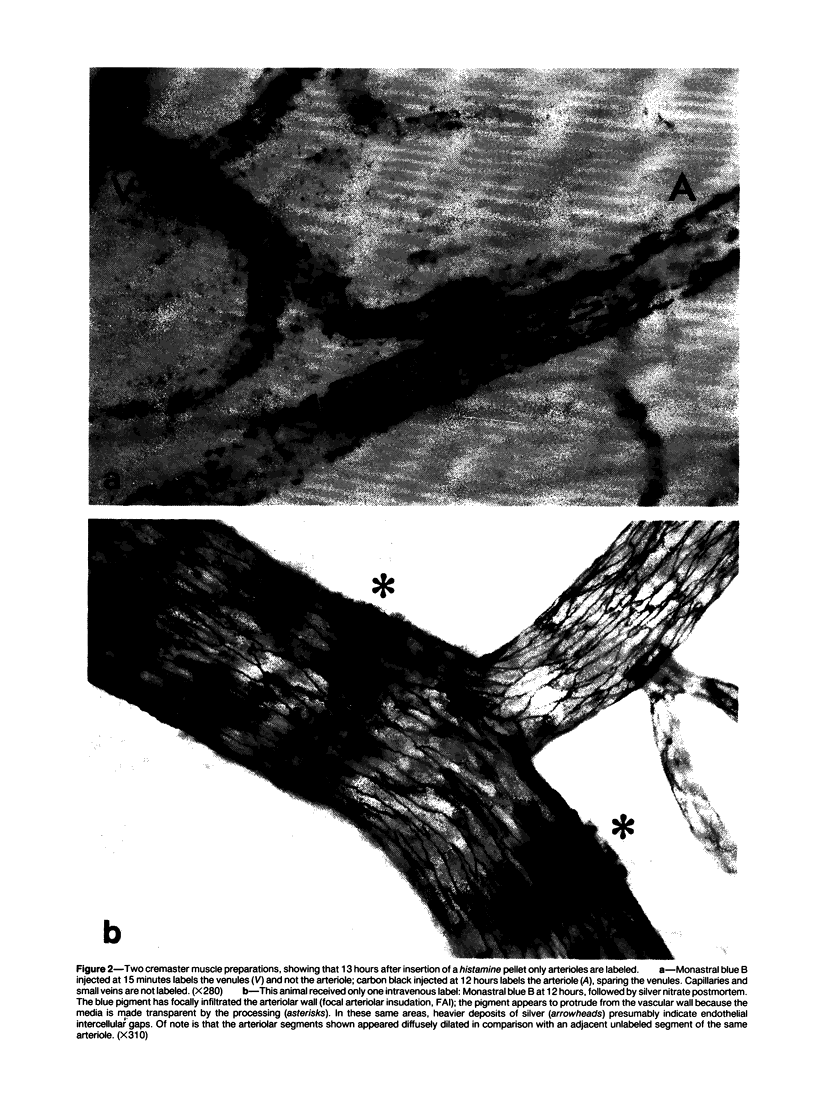
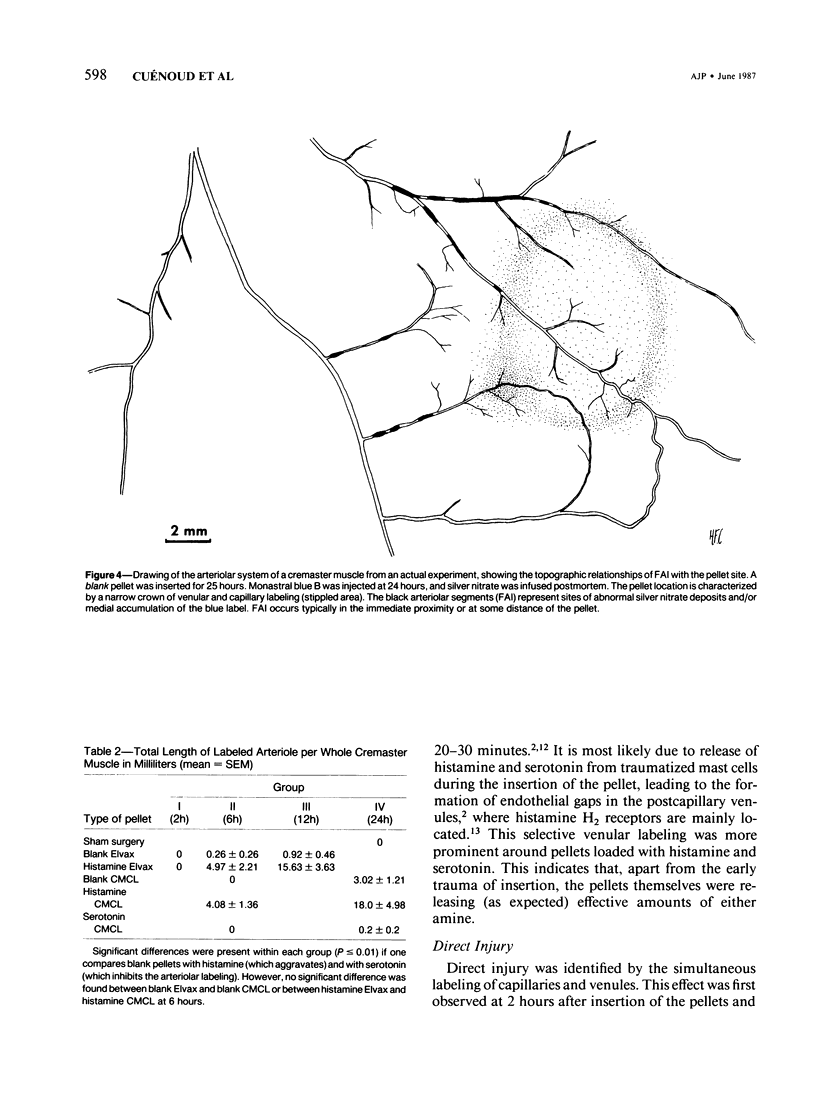
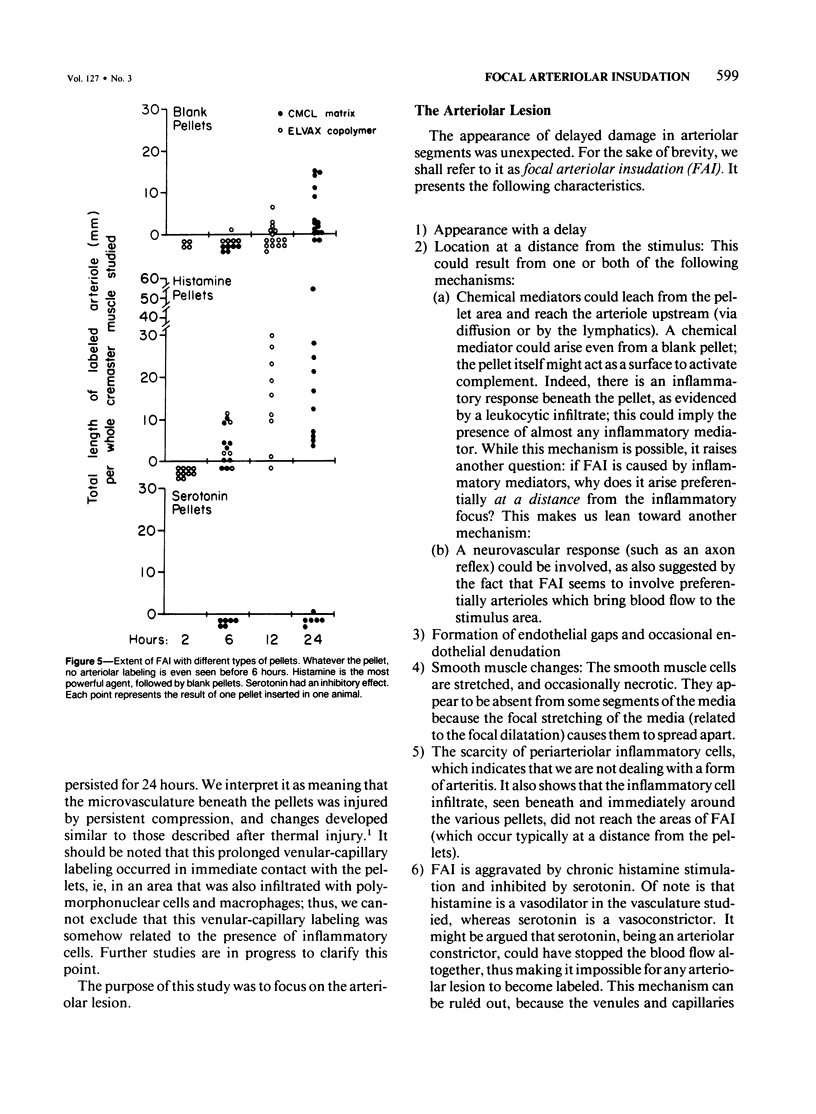
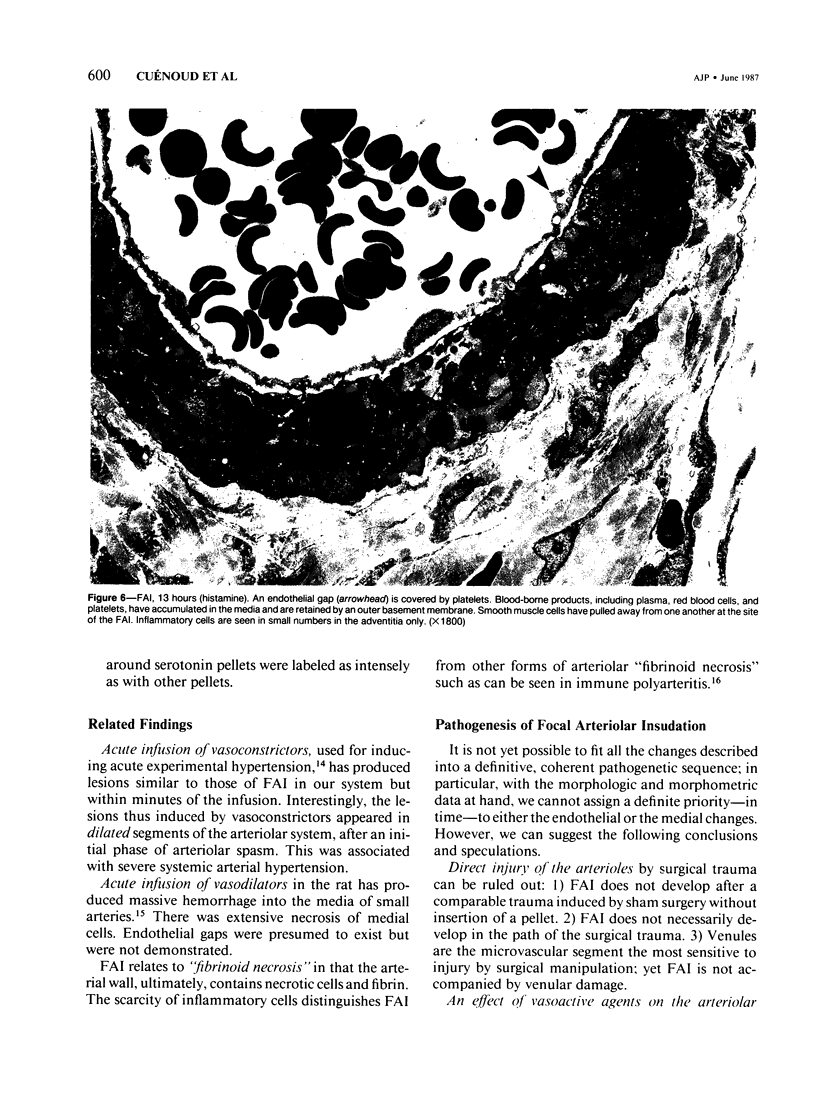
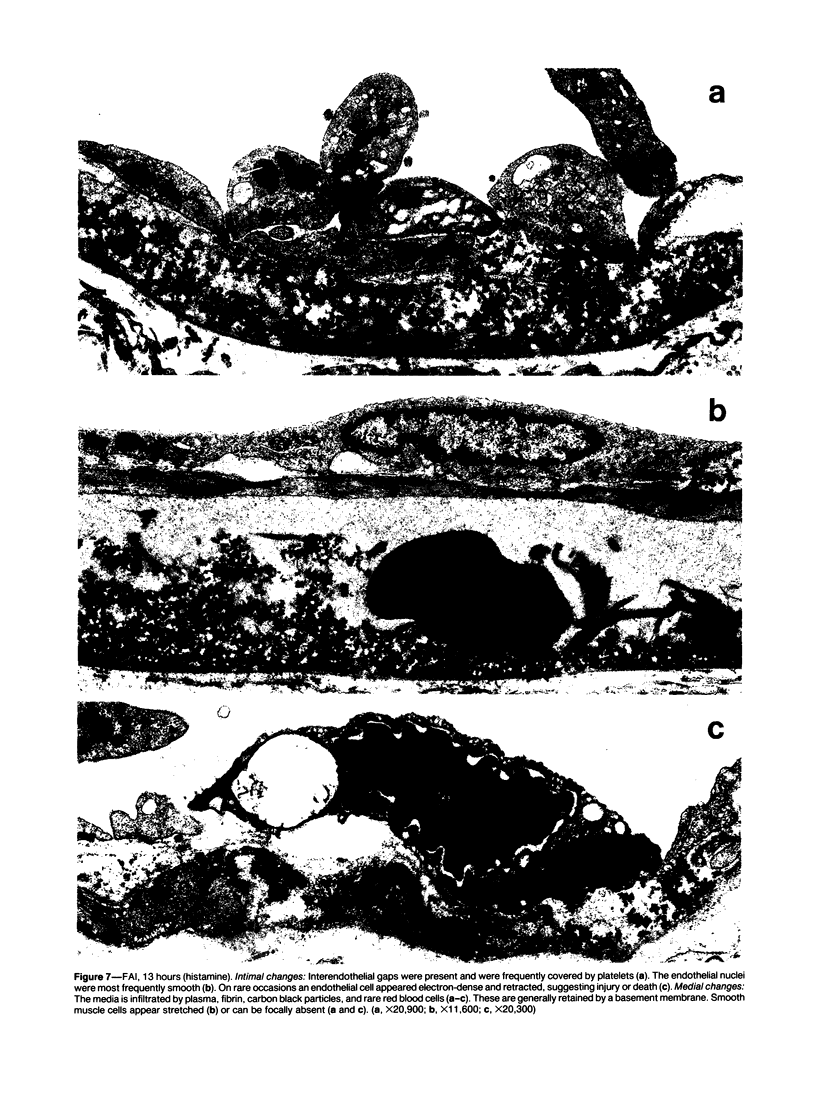
The subcutaneous insertion of sterile, inert plastic pellets over the cremaster muscles of rats induces characteristic focal lesions of the arterioles at a distance from the pellets. These lesions appear with a delay of about 6 hours; by light microscopy they are characterized by a focal dilatation accompanied by endothelial damage and increased permeability. They are more severe if the pellets are loaded with histamine and are inhibited if the pellets are loaded with serotonin. Electron microscopy shows interendothelial gaps; the media is massively infiltrated with blood components and fibrin. The medial smooth muscle cells are stretched and at times necrotic; inflammatory cells are scarce. On the basis of these features the lesion was named focal arteriolar insudation (FAI). Although its pathogenesis is not yet clear, the data at hand suggest that it is caused by endogenous mediators affecting the smooth muscle cells and/or the endothelium. FAI appears to be a specific arteriolar response to chronic nonspecific irritation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch C., Gerdin B. Effect of low molecular weight fibrin degradation products on endothelial cells in culture. Thromb Res. 1981 Apr 1;22(1-2):33–39. doi: 10.1016/0049-3848(81)90306-6. [DOI] [PubMed] [Google Scholar]
- Conn D. L., McDuffie F. C., Holley K. E., Schroeter A. L. Immunologic mechanisms in systemic vasculitis. Mayo Clin Proc. 1976 Aug;51(8):511–518. [PubMed] [Google Scholar]
- Cotran R. S., Remensnyder J. P. The structural basis of increased vascular permeabiligy after graded thermal injury--light and electron microscopic studies. Ann N Y Acad Sci. 1968 Aug 14;150(3):495–509. doi: 10.1111/j.1749-6632.1968.tb14702.x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Bell W. R., Kaiser D., Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985 Mar 22;227(4693):1487–1490. doi: 10.1126/science.4038818. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- GIESE J. ACUTE HYPERTENSIVE VASCULAR DISEASE. 2. STUDIES ON VASCULAR REACTION PATTERNS AND PERMEABILITY CHANGES BY MEANS OF VITAL MICROSCOPY AND COLLOIDAL TRACER TECHNIQUE. Acta Pathol Microbiol Scand. 1964;62:497–515. doi: 10.1111/apm.1964.62.4.497. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C., Majno G. Intra-arterial injections of histamine, serotonin, or bradykinin: a topographic study of vascular leakage. Proc Soc Exp Biol Med. 1970 Nov;135(2):447–452. doi: 10.3181/00379727-135-35072. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Buchanan M. R. Interactions of platelets and leukocytes with vascular endothelium: in vitro studies. Ann N Y Acad Sci. 1982;401:171–183. doi: 10.1111/j.1749-6632.1982.tb25716.x. [DOI] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Heltianu C., Simionescu M., Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982 May;93(2):357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan K. L., Adamski S. W., Ayele W., Langone J. J., Grega G. J. Evidence that prolonged histamine suffusions produce transient increases in vascular permeability subsequent to the formation of venular macromolecular leakage sites. Proof of the Majno-Palade hypothesis. Am J Pathol. 1986 Jun;123(3):570–576. [PMC free article] [PubMed] [Google Scholar]
- Joris I., DeGirolami U., Wortham K., Majno G. Vascular labelling with monastral blue B. Stain Technol. 1982 May;57(3):177–183. doi: 10.3109/10520298209066611. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G. Endothelial changes induced by arterial spasm. Am J Pathol. 1981 Mar;102(3):346–358. [PMC free article] [PubMed] [Google Scholar]
- Kadish J. L., Butterfield C. E., Folkman J. The effect of fibrin on cultured vascular endothelial cells. Tissue Cell. 1979;11(1):99–108. doi: 10.1016/0040-8166(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Brown L., Edelman E. Controlled release and magnetically modulated release systems for macromolecules. Methods Enzymol. 1985;112:399–422. doi: 10.1016/s0076-6879(85)12032-x. [DOI] [PubMed] [Google Scholar]
- Langer R., Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976 Oct 28;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Increased permeability associated with dilatation of endothelial cell junctions caused by histamine in intimal explants from bovine pulmonary artery. Exp Lung Res. 1984;6(1):11–25. doi: 10.3109/01902148409087892. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland F. N., Donovan M. J., Picciano P. T., Wilner G. D., Kreutzer D. L. Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol. 1984 Dec;117(3):418–428. [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Tanaka K. Influence of fibrin, fibrinogen and fibrinogen degradation products on cultured endothelial cells. Atherosclerosis. 1983 Jul;48(1):57–70. doi: 10.1016/0021-9150(83)90017-5. [DOI] [PubMed] [Google Scholar]
- Yuhas E. M., Morgan D. G., Arena E., Kupp R. P., Saunders L. Z., Lewis H. B. Arterial medial necrosis and hemorrhage induced in rats by intravenous infusion of fenoldopam mesylate, a dopaminergic vasodilator. Am J Pathol. 1985 Apr;119(1):83–91. [PMC free article] [PubMed] [Google Scholar]
- Zand T., Underwood J. M., Nunnari J. J., Majno G., Joris I. Endothelium and "silver lines". An electron microscopic study. Virchows Arch A Pathol Anat Histol. 1982;395(2):133–144. doi: 10.1007/BF00429607. [DOI] [PubMed] [Google Scholar]