Abstract
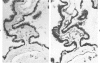
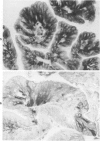
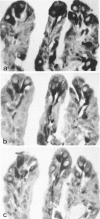
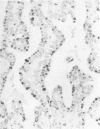
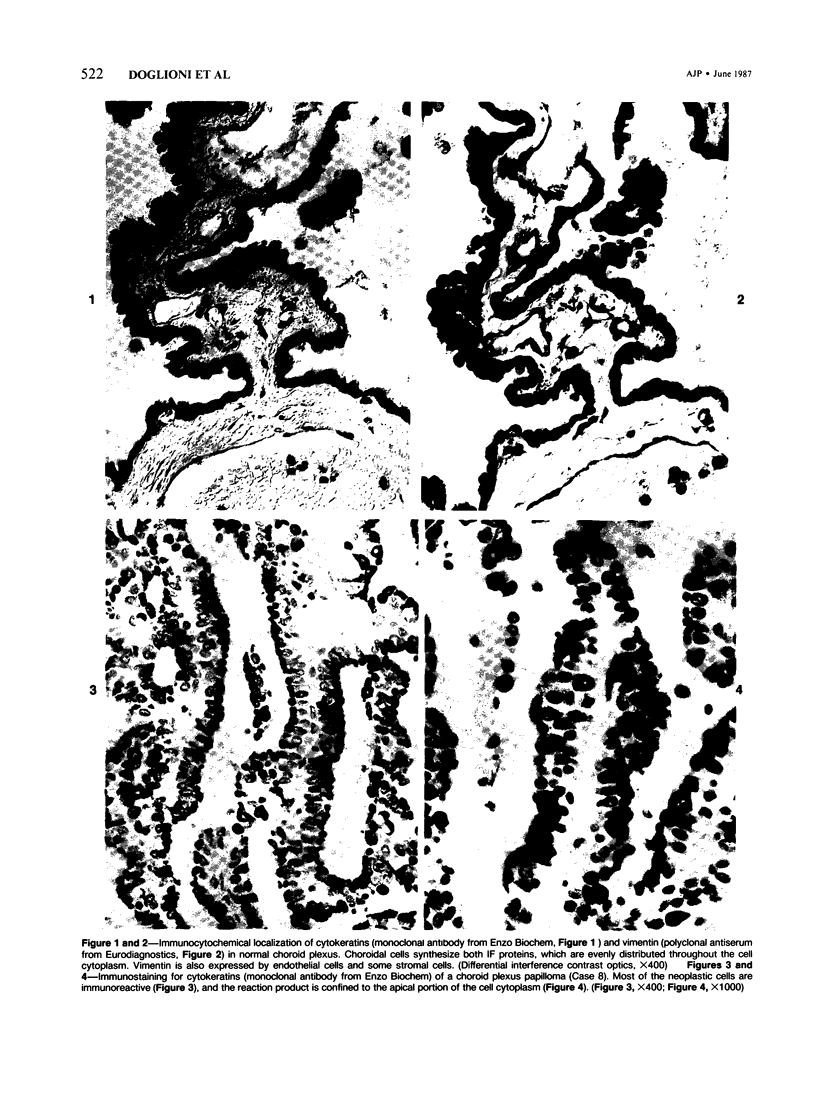
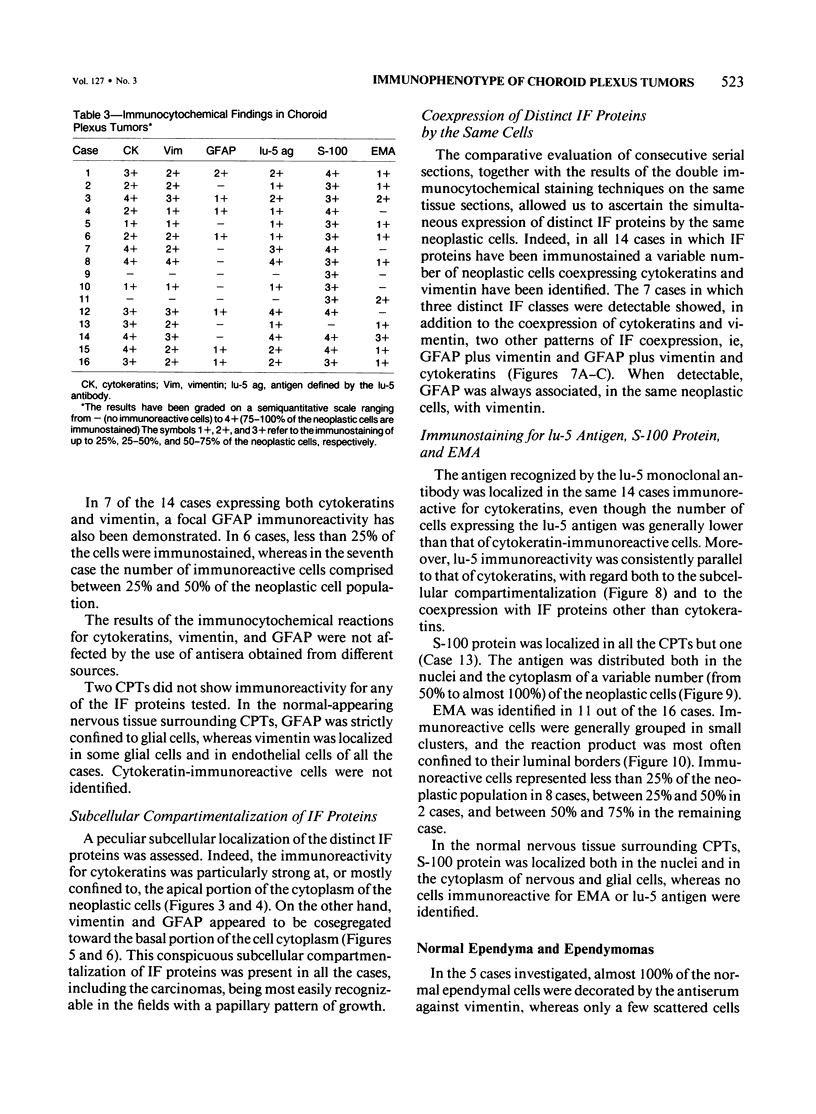
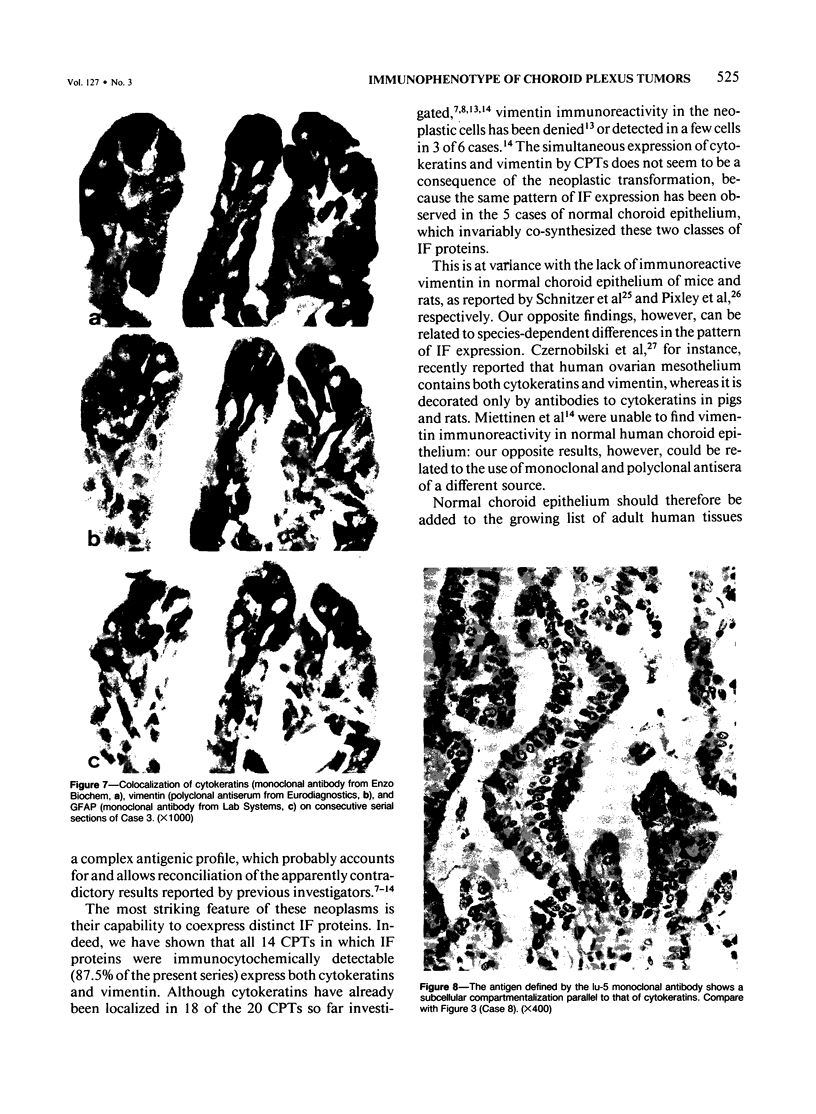
Sixteen choroid plexus tumors (CPTs) have been investigated for the localization of different immunocytochemical markers of epithelial and nonepithelial nature, namely, simple epithelial-type cytokeratins, vimentin, glial fibrillary acidic protein (GFAP), a panepithelial antigen defined by the lu-5 monoclonal antibody (lu-5 antigen), S-100 protein, and epithelial membrane antigen (EMA). Intermediate filament proteins have been identified in paraffin sections of 14 of 16 cases (87.5%). In all these tumors, cytokeratins and vimentin were constantly coexpressed by the neoplastic cells, in a manner similar to that of the cells lining normal choroid plexus. In 7 of these 14 cases, in addition to cytokeratins and vimentin, the neoplastic cells were shown to coexpress GFAP, which is not synthesized by their normal cell counterpart. The appearance of GFAP immunoreactivity in CPTs might be related to an ependymal differentiation of the neoplastic cells, because normal ependyma and ependymomas constantly coexpress GFAP and vimentin. The simultaneous expression of three distinct intermediate filament proteins by the same neoplastic cells is an exceedingly rare phenomenon, which has never been reported by double labeling technique in neoplasms of the central nervous system. Despite the complex antigenic profile of the CPT, which includes immunoreactivity for lu-5 antigen, S-100 protein, and EMA in most of the cases, positivity for three different epithelial markers indicates that these tumors have an epithelial nature. Moreover, the immunocytochemical typing of CPT with the panel of antibodies used in the current investigation allows differentiation from other primary and metastatic central nervous system tumors.
Full text
PDF



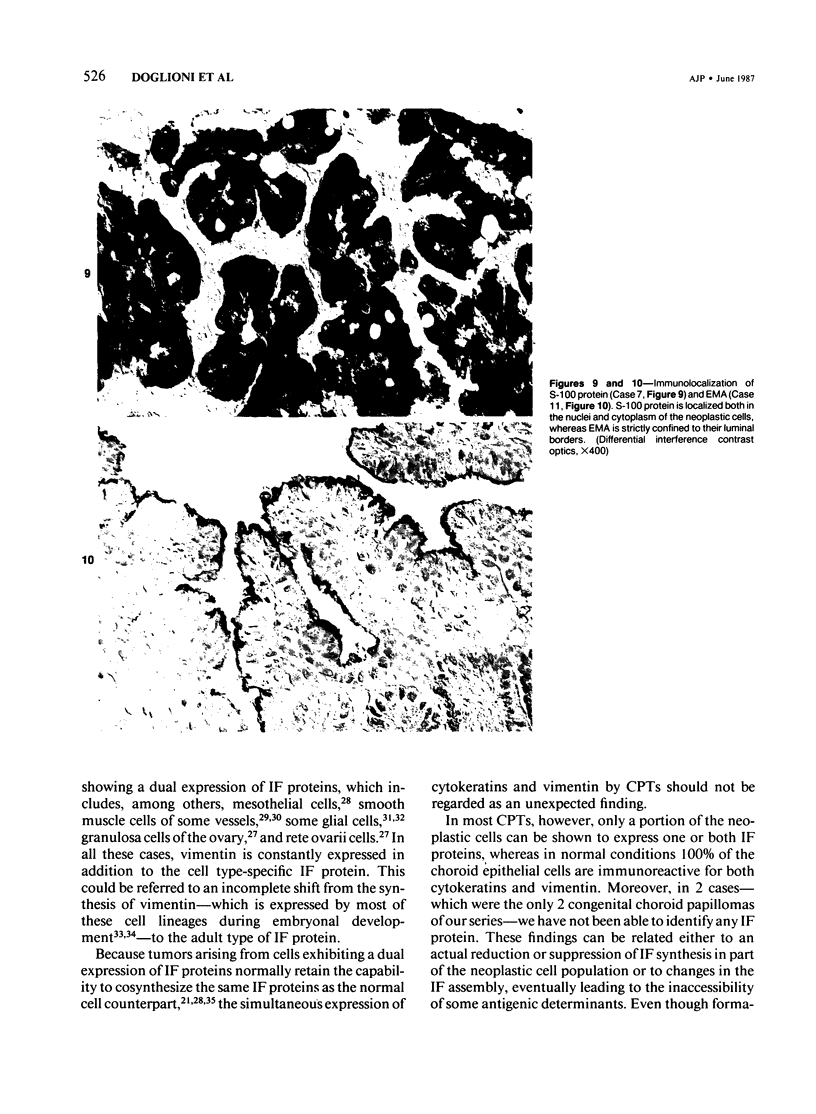



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstätter T., Moll R., Anderson A., Kuhn C., Pitz S., Schwechheimer K., Franke W. W. Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation. 1986;31(3):206–227. doi: 10.1111/j.1432-0436.1986.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Fernandez E. Intracytoplasmic fibrillary inclusions in bronchial carcinoid. Cancer. 1980 Jul 1;46(1):144–151. doi: 10.1002/1097-0142(19800701)46:1<144::aid-cncr2820460123>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- An T. Cytoplasmic inclusions in bronchial carcinoid. Hum Pathol. 1978 Mar;9(2):241–242. doi: 10.1016/s0046-8177(78)80121-x. [DOI] [PubMed] [Google Scholar]
- Anguilar D., Martín J. M., Aneiros J., Arjona V., Lara J. L., Nogales F. The fine structure of choroid plexus carcinoma. Histopathology. 1983 Nov;7(6):939–946. doi: 10.1111/j.1365-2559.1983.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Berger G., Berger F., Bejui F., Bouvier R., Rochet M., Feroldi J. Bronchial carcinoid with fibrillary inclusions related to cytokeratins: an immunohistochemical and ultrastructural study with subsequent investigation of 12 foregut APUDomas. Histopathology. 1984 Mar;8(2):245–257. doi: 10.1111/j.1365-2559.1984.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Blobel G. A., Moll R., Franke W. W., Kayser K. W., Gould V. E. The intermediate filament cytoskeleton of malignant mesotheliomas and its diagnostic significance. Am J Pathol. 1985 Nov;121(2):235–247. [PMC free article] [PubMed] [Google Scholar]
- Carter L. P., Beggs J., Waggener J. D. Ultrastructure of three choroid plexus papillomas. Cancer. 1972 Oct;30(4):1130–1136. doi: 10.1002/1097-0142(197210)30:4<1130::aid-cncr2820300434>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Coakham H. B., Garson J. A., Allan P. M., Harper E. I., Brownell B., Kemshead J. T., Lane E. B. Immunohistological diagnosis of central nervous system tumours using a monoclonal antibody panel. J Clin Pathol. 1985 Feb;38(2):165–173. doi: 10.1136/jcp.38.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin C. M., Wick M. R., Braun J. T., Dehner L. P. Choroid plexus neoplasms. Clinicopathologic and immunohistochemical studies. Am J Surg Pathol. 1986 Jun;10(6):394–404. doi: 10.1097/00000478-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Czernobilsky B., Moll R., Levy R., Franke W. W. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol. 1985 May;37:175–190. [PubMed] [Google Scholar]
- Dahl D., Rueger D. C., Bignami A., Weber K., Osborn M. Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol. 1981 Jun;24(2):191–196. [PubMed] [Google Scholar]
- Damjanov I. Antibodies to intermediate filaments and histogenesis. Lab Invest. 1982 Sep;47(3):215–217. [PubMed] [Google Scholar]
- Duffy P. E., Graf L., Huang Y. Y., Rapport M. M. Glial fibrillary acidic protein in ependymomas and other brain tumors. Distribution, diagnostic criteria, and relation to formation of processes. J Neurol Sci. 1979 Feb;40(2-3):133–146. doi: 10.1016/0022-510x(79)90199-0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Anti-intermediate filament monoclonal antibodies: tissue-specific tools in tumor diagnosis. Surv Synth Pathol Res. 1984;3(5):369–385. doi: 10.1159/000156940. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Herman C. J., Vegt P. D., Debruyne F. M., Vooijs G. P., Ramaekers F. C. Squamous and transitional elements in rat bladder carcinomas induced by N-butyl-N-4-hydroxybutyl-nitrosamine (BBN). A study of cytokeratin expression. Am J Pathol. 1985 Sep;120(3):419–426. [PMC free article] [PubMed] [Google Scholar]
- Horvath E., Kovacs K. Morphogenesis and significance of fibrous bodies in human pituitary adenomas. Virchows Arch B Cell Pathol. 1978 Mar 2;27(1):69–78. doi: 10.1007/BF02888984. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kemshead J. T., Coakham H. B. The use of monoclonal antibodies for the diagnosis of intracranial malignancies and the small round cell tumours of childhood. J Pathol. 1983 Nov;141(3):249–257. doi: 10.1002/path.1711410306. [DOI] [PubMed] [Google Scholar]
- Kimura T., Budka H., Soler-Federsppiel S. An immunocytochemical comparison of the glia-associated proteins glial fibrillary acidic protein (GFAP) and S-100 protein (S100P) in human brain tumors. Clin Neuropathol. 1986 Jan-Feb;5(1):21–27. [PubMed] [Google Scholar]
- LaRocca P. J., Rheinwald J. G. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 1984 Jul;44(7):2991–2999. [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Clark R., Virtanen I. Intermediate filament proteins in choroid plexus and ependyma and their tumors. Am J Pathol. 1986 May;123(2):231–240. [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Becker L. E., Marks A. Distribution of immunoreactive S-100 protein in pediatric brain tumors. J Neuropathol Exp Neurol. 1983 Mar;42(2):136–145. doi: 10.1097/00005072-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Nakashima N., Goto K., Tsukidate K., Sobue M., Toida M., Takeuchi J. Choroid plexus papilloma. Light and electron microscopic study. Virchows Arch A Pathol Anat Histopathol. 1983;400(2):201–211. doi: 10.1007/BF00585501. [DOI] [PubMed] [Google Scholar]
- Navas J. J., Battifora H. Choroid plexus papilloma: light and electron microscopic study of three cases. Acta Neuropathol. 1978 Dec 15;44(3):235–239. doi: 10.1007/BF00691073. [DOI] [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Pasquier B., Lachard A., Pasquier D., Couderc P., Delpech B., Courel M. N. Protéine gliofibrillaire acide (GFA) et tumeurs nerveuses centrales. Etude immunohistochimique d'une série de 207 cas. 1re partie: Astrocytomes. Glioblastomes. Ependymomes. Papillomes des plexus choroïdes. Ann Pathol. 1983;3(2):127–135. [PubMed] [Google Scholar]
- Pinkus G. S., Kurtin P. J. Epithelial membrane antigen--a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol. 1985 Sep;16(9):929–940. doi: 10.1016/s0046-8177(85)80132-5. [DOI] [PubMed] [Google Scholar]
- Pixley S. K., Kobayashi Y., de Vellis J. A monoclonal antibody against vimentin: characterization. Brain Res. 1984 Aug;317(2):185–199. doi: 10.1016/0165-3806(84)90096-8. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Brucher J. M. Focal ependymal differentiation in choroid plexus papillomas. An immunoperoxidase study. Acta Neuropathol. 1981;53(1):29–33. doi: 10.1007/BF00697181. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S., Dockhorn-Dworniczak B., Kastendieck H., Böcker W., Franke W. W. Intermediate-filament expression in thyroid gland carcinomas. Virchows Arch A Pathol Anat Histopathol. 1986;409(6):751–766. doi: 10.1007/BF00710761. [DOI] [PubMed] [Google Scholar]
- Sibley R. K., Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. An immunocytochemical study of 21 cases. Am J Surg Pathol. 1985 Feb;9(2):109–116. doi: 10.1097/00000478-198502000-00005. [DOI] [PubMed] [Google Scholar]
- Sibley R. K., Dehner L. P., Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. A clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985 Feb;9(2):95–108. doi: 10.1097/00000478-198502000-00004. [DOI] [PubMed] [Google Scholar]
- Sloane J. P., Ormerod M. G. Distribution of epithelial membrane antigen in normal and neoplastic tissues and it value in diagnostic tumor pathology. Cancer. 1981 Apr 1;47(7):1786–1795. doi: 10.1002/1097-0142(19810401)47:7<1786::aid-cncr2820470711>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Stefanko S. Z., Vuzevski V. D. Oncocytic variant of choroid plexus papilloma. Acta Neuropathol. 1985;66(2):160–162. doi: 10.1007/BF00688692. [DOI] [PubMed] [Google Scholar]
- Taratuto A. L., Molina H., Monges J. Choroid plexus tumors in infancy and childhood. Focal ependymal differentiation. An immunoperoxidase study. Acta Neuropathol. 1983;59(4):304–308. doi: 10.1007/BF00691497. [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Miettinen M., Lehto V. P., Kariniemi A. L., Paasivuo R. Diagnostic application of monoclonal antibodies to intermediate filaments. Ann N Y Acad Sci. 1985;455:635–648. doi: 10.1111/j.1749-6632.1985.tb50441.x. [DOI] [PubMed] [Google Scholar]
- Wang E., Cairncross J. G., Liem R. K. Identification of glial filament protein and vimentin in the same intermediate filament system in human glioma cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2102–2106. doi: 10.1073/pnas.81.7.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]