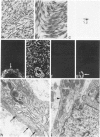
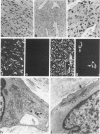
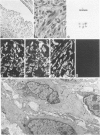
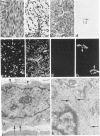
Abstract
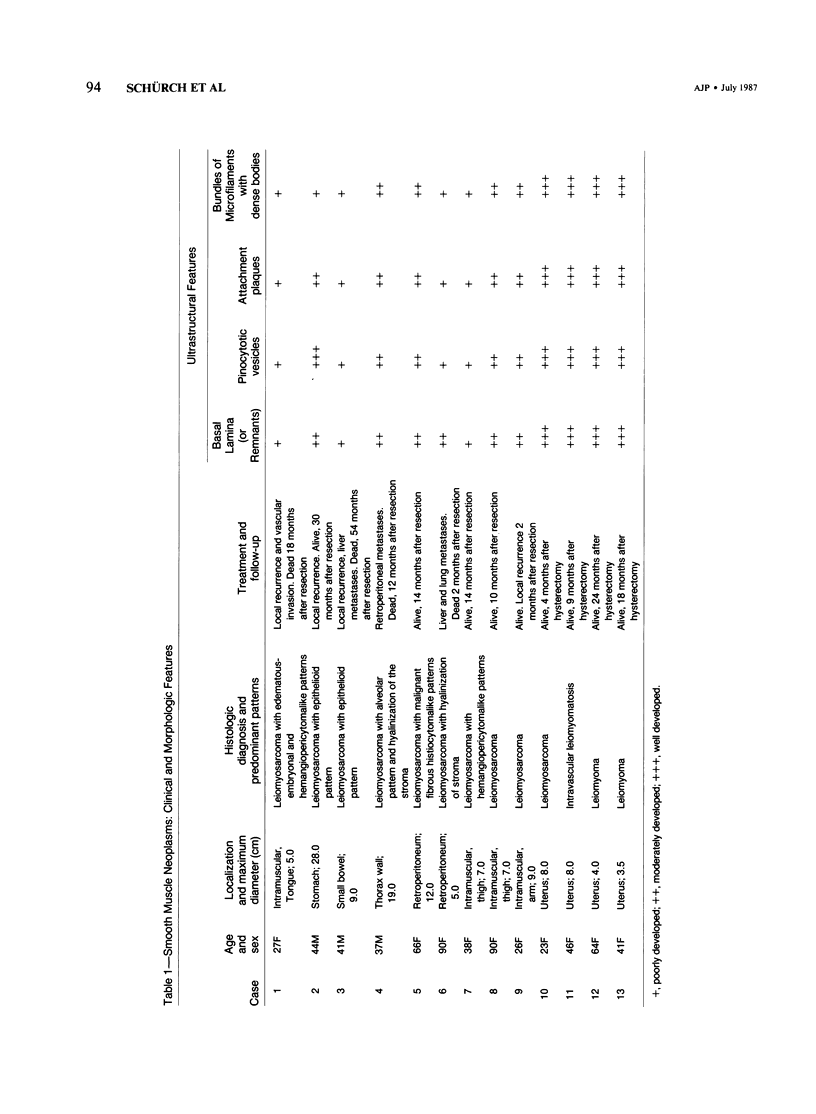
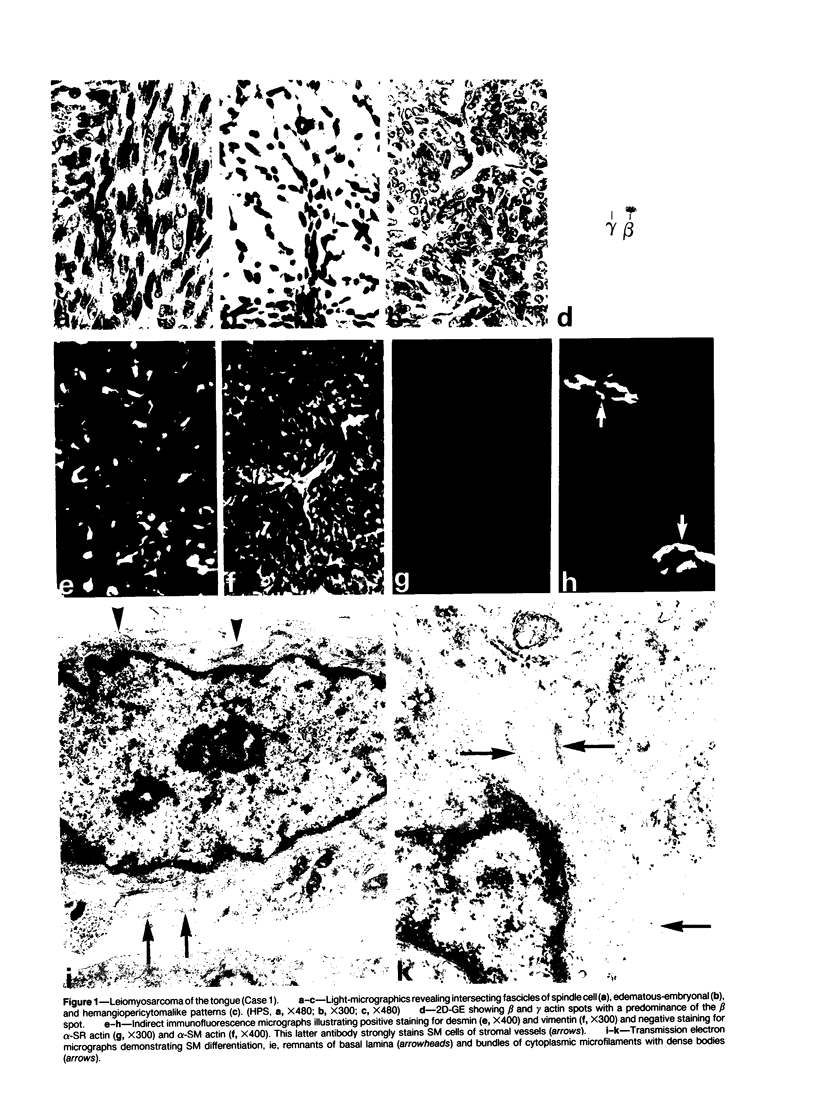
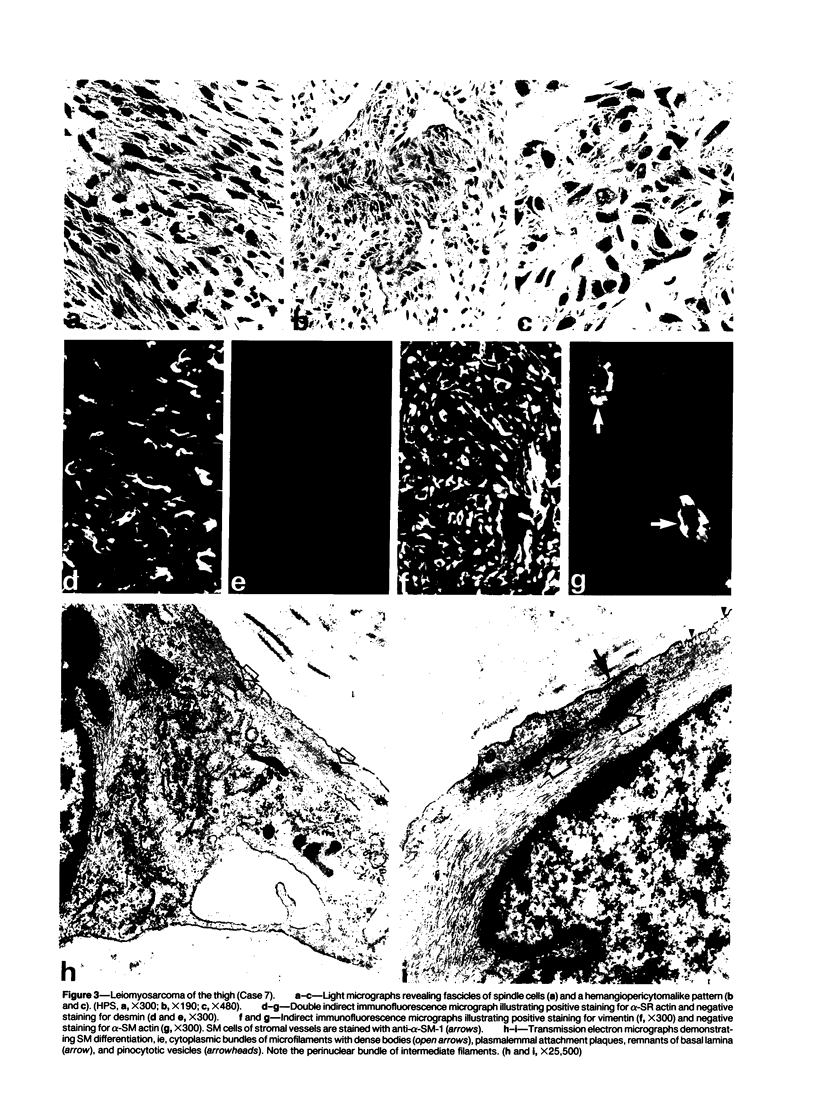
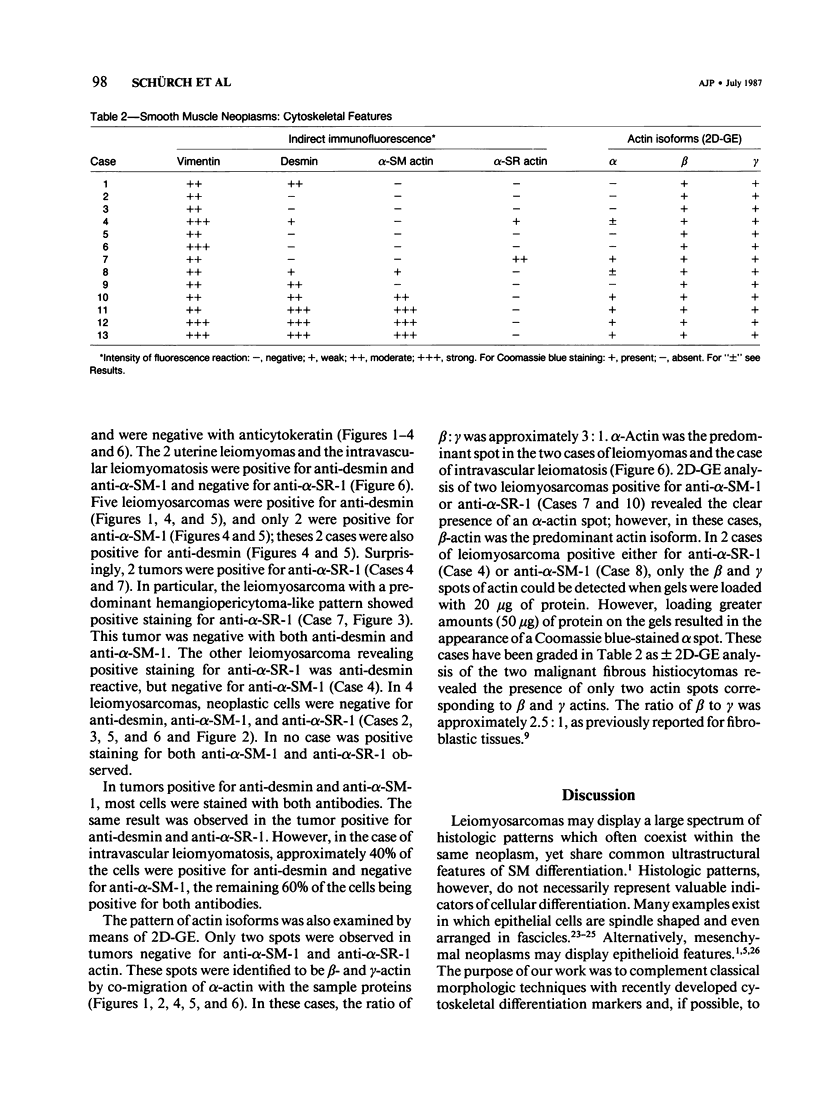
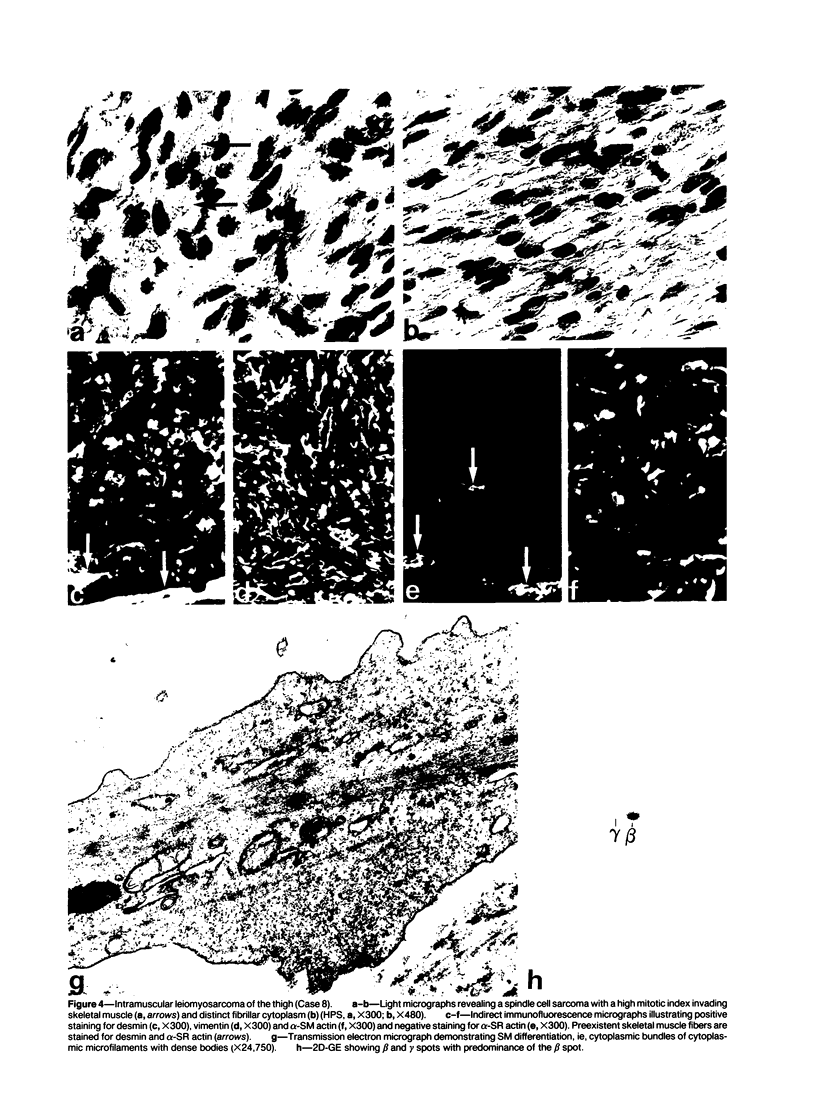
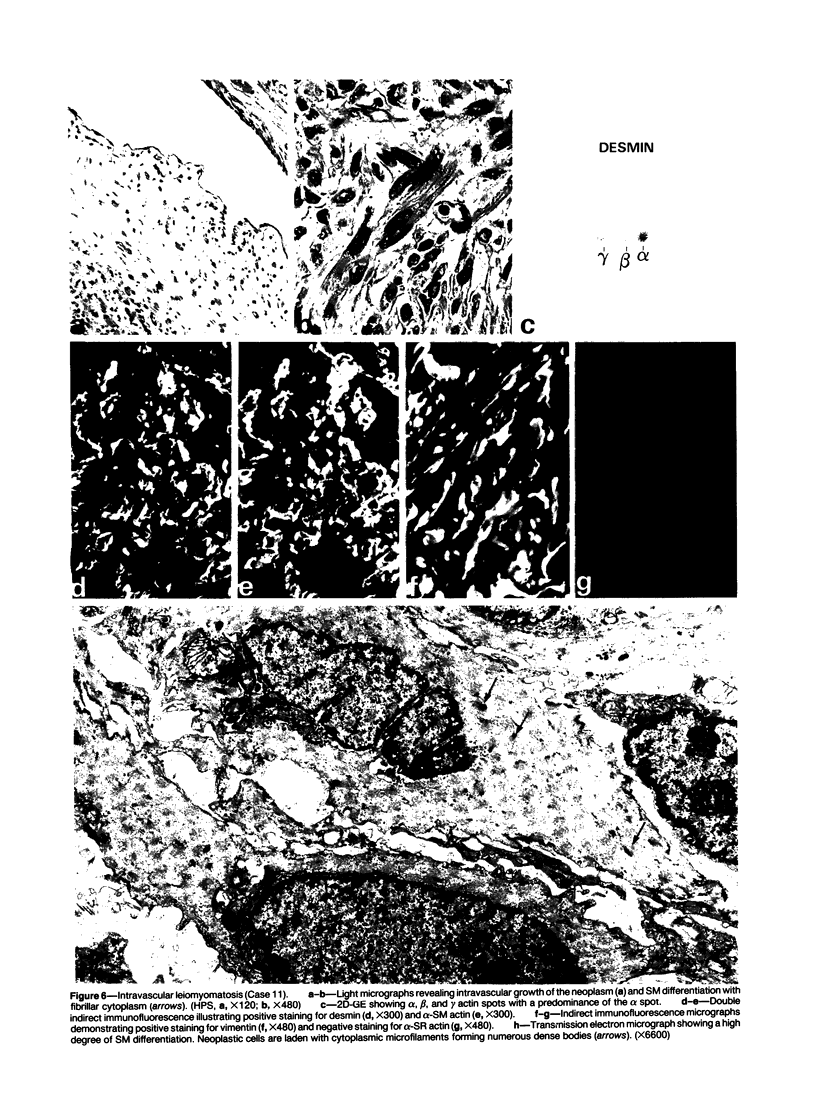
A series of 3 benign and 10 malignant smooth muscle (SM) neoplasms and of 2 malignant fibrous histiocytomas was examined by light microscopy, transmission electron microscopy, two-dimensional gel electrophoresis (2D-GE) and indirect immunofluorescence, using polyclonal monospecific or monoclonal antibodies to desmin, vimentin, cytokeratin, alpha-SM and alpha-sarcomeric (alpha-SR) actins. Benign neoplasms displayed typical light-microscopic features of SM, whereas leiomyosarcomas demonstrated variations in their histologic pattern. In 6 sarcomas, light microscopy suggested a SM differentiation, whereas in the other 4, a predominant nondistinctive spindle-cell pattern was observed. By transmission electron microscopy, all 13 neoplasms showed the minimal essential features of SM differentiation. Immunofluorescence disclosed heterogeneity of cytoskeletal protein expression: 5 neoplasms (3 benign and 2 malignant well-differentiated) expressed desmin, vimentin, and alpha-SM-actin; 2 malignant neoplasms expressed desmin and vimentin; 1 malignant neoplasm expressed desmin, vimentin and alpha-SR actin; 1 malignant neoplasm expressed vimentin and alpha-SR actin; and 4 malignant neoplasms expressed vimentin alone. By 2D-GE, 3 benign and 4 malignant SM neoplasms expressed alpha, beta, and gamma actins, and the remaining expressed only beta and gamma actins. The presence of alpha-SM actin in all benign neoplasms and in 2 well-differentiated leiomyosarcomas suggests that this actin isoform reflects a high degree of cellular differentiation. In 2 leiomyosarcomas, alpha-SR actin was detected by immunofluorescence, which suggested a skeletal muscle differentiation of these neoplasms. This study supports the assumption that leiomyosarcomas represent a heterogeneous group of neoplasms and furnishes new criteria for their characterization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Schauer A., Weber K. Antibodies to different intermediate filament proteins. Cell type-specific markers on paraffin-embedded human tissues. Lab Invest. 1981 Nov;45(5):427–434. [PubMed] [Google Scholar]
- Altmannsberger M., Weber K., Droste R., Osborn M. Desmin is a specific marker for rhabdomyosarcomas of human and rat origin. Am J Pathol. 1985 Jan;118(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Bannasch P., Zerban H., Schmid E., Franke W. W. Liver tumors distinguished by immunofluorescence microscopy with antibodies to proteins of intermediate-sized filaments. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4948–4952. doi: 10.1073/pnas.77.8.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer T. W., Rostock R. A., Eggleston J. C., Baral E. Spindle cell carcinoma of the breast: four cases and review of the literature. Hum Pathol. 1984 Feb;15(2):147–152. doi: 10.1016/s0046-8177(84)80055-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Chase D. R., Enzinger F. M., Weiss S. W., Langloss J. M. Keratin in epithelioid sarcoma. An immunohistochemical study. Am J Surg Pathol. 1984 Jun;8(6):435–441. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- Denk H., Krepler R., Artlieb U., Gabbiani G., Rungger-Brändle E., Leoncini P., Franke W. W. Proteins of intermediate filaments. An immunohistochemical and biochemical approach to the classification of soft tissue tumors. Am J Pathol. 1983 Feb;110(2):193–208. [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Lampert I. A., Jacobs M. Intermediate filaments in smooth muscle tumours. J Clin Pathol. 1983 Jan;36(1):57–61. doi: 10.1136/jcp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. D., Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci U S A. 1981 May;78(5):3020–3024. doi: 10.1073/pnas.78.5.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. S., Gabbiani G., Kaye G. I., Lattes R. Malignant soft tissue tumors of probable histiocytic origin (malignant fibrous histiocytomas): general considerations and electron microscopic and tissue culture studies. Cancer. 1975 Jan;35(1):176–198. doi: 10.1002/1097-0142(197501)35:1<176::aid-cncr2820350123>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Rungger-Brändle E., de Chastonay C., Franke W. W. Vimentin-containing smooth muscle cells in aortic intimal thickening after endothelial injury. Lab Invest. 1982 Sep;47(3):265–269. [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersell D. J., Katzenstein A. L. Spindle cell carcinoma of the breast. A clinocopathologic and ultrastructural study. Hum Pathol. 1981 Jun;12(6):550–561. doi: 10.1016/s0046-8177(81)80069-x. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lennette D. A. An improved mounting medium for immunofluorescence microscopy. Am J Clin Pathol. 1978 Jun;69(6):647–648. doi: 10.1093/ajcp/69.6.647. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Antibodies to intermediate filament proteins in the diagnosis and classification of human tumors. Ultrastruct Pathol. 1984;7(2-3):83–107. doi: 10.3109/01913128409141467. [DOI] [PubMed] [Google Scholar]
- Norris H. J., Parmley T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis. A clinical and pathologic study of 14 cases. Cancer. 1975 Dec;36(6):2164–2178. doi: 10.1002/cncr.2820360935. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Altmannsberger M., Debus E., Weber K. Differentiation of the major human tumor groups using conventional and monoclonal antibodies specific for individual intermediate filament proteins. Ann N Y Acad Sci. 1985;455:649–668. doi: 10.1111/j.1749-6632.1985.tb50442.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. doi: 10.1111/j.1432-0436.1981.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Schmid E., Osborn M., Rungger-Brändle E., Gabbiani G., Weber K., Franke W. W. Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Exp Cell Res. 1982 Feb;137(2):329–340. doi: 10.1016/0014-4827(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Schmid E., Tapscott S., Bennett G. S., Croop J., Fellini S. A., Holtzer H., Franke W. W. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation. 1979;15(1):27–40. doi: 10.1111/j.1432-0436.1979.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Schürch W., Potvin C., Seemayer T. A. Malignant myoepithelioma (myoepithelial carcinoma) of the breast: an ultrastructural and immunocytochemical study. Ultrastruct Pathol. 1985;8(1):1–11. doi: 10.3109/01913128509141504. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Weber K., Osborn M. Co-existence of vimentin and desmin type intermediate filaments in a subpopulation of adult rat vascular smooth muscle cells growing in primary culture. Exp Cell Res. 1982 May;139(1):87–94. doi: 10.1016/0014-4827(82)90321-4. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin typing on total cellular extracts: a highly sensitive protein-chemical procedure able to distinguish different actins. Eur J Biochem. 1981 Jan;113(3):595–603. doi: 10.1111/j.1432-1033.1981.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]