Abstract
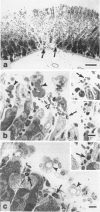
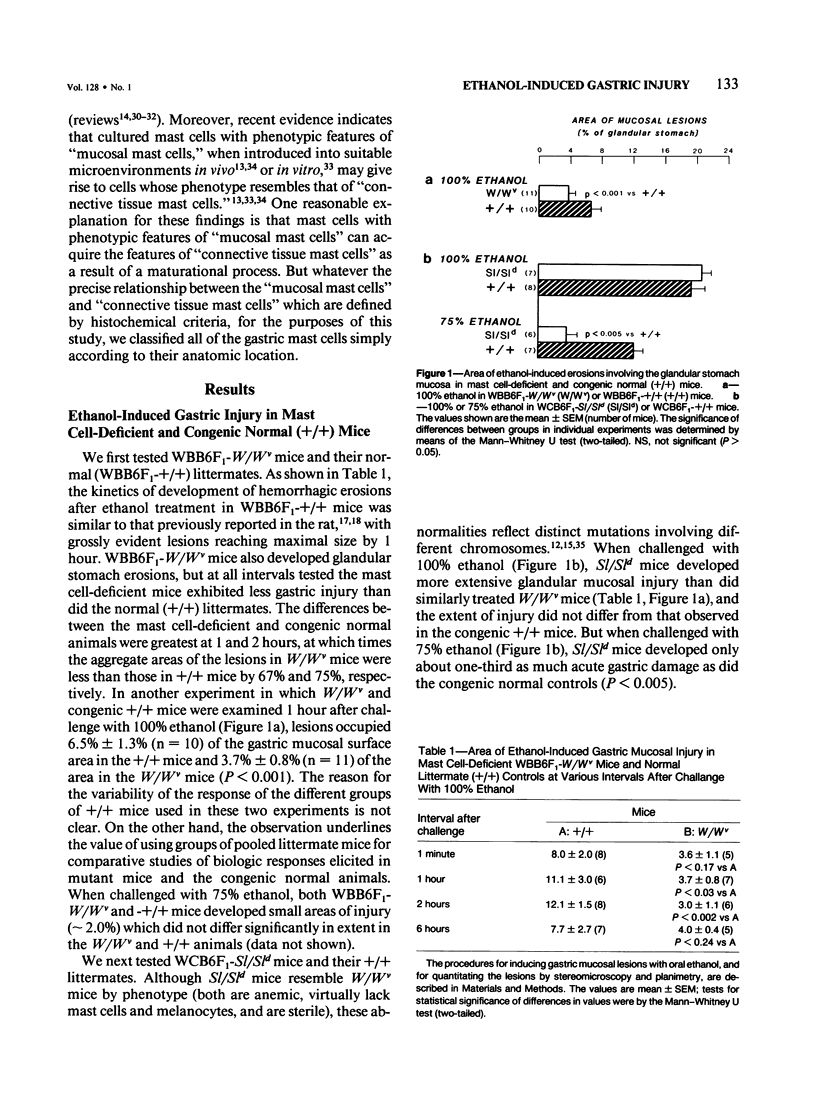
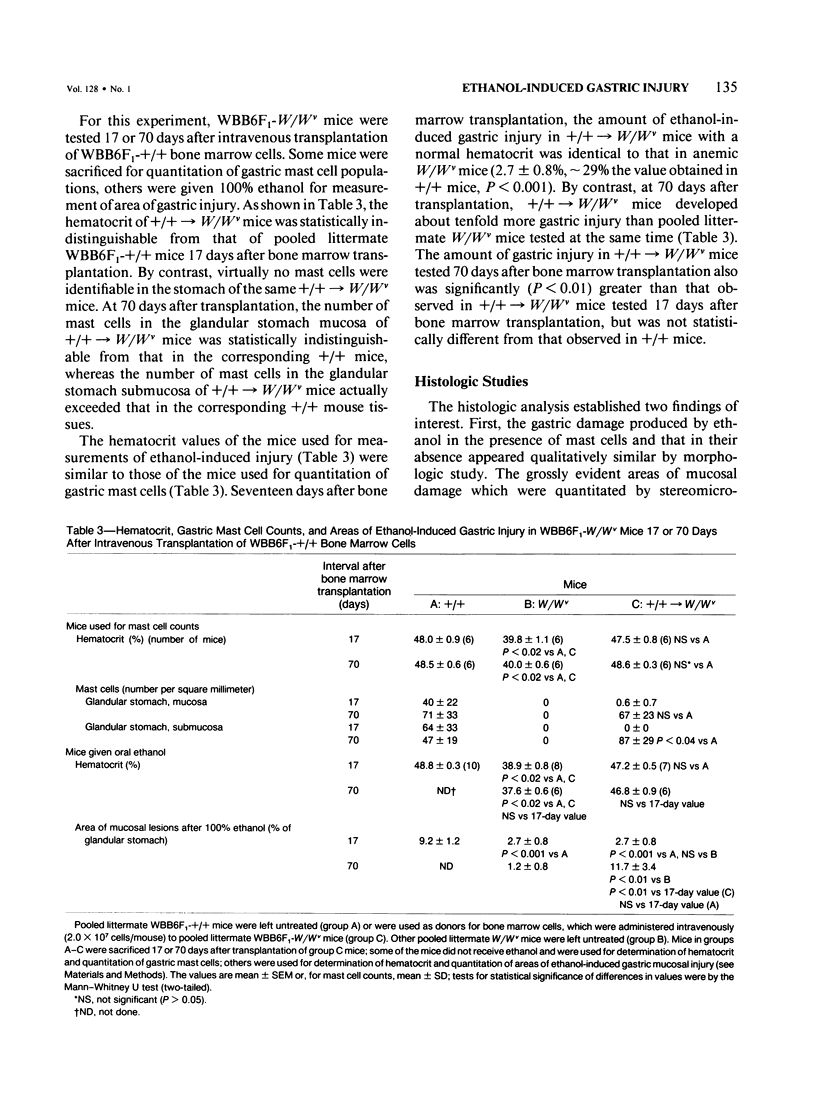
The authors used stereomicroscopy and planimetry to measure the area of glandular stomach mucosa acutely injured by oral ethanol in mast cell-deficient and congenic normal (+/+) mice, and examined the damaged areas in 1-mu sections. Ethanol caused degranulation and/or disruption of gastric mucosal mast cells, and, at certain concentrations of ethanol, mast cell-deficient WBB6F1-W/Wv or WCB6F1-Sl/Sld mice developed significantly less (43-90% less) acute gastric injury than either congenic +/+ mice or WBB6F1-W/Wv mice whose mast cells were restored by bone marrow transplantation from WBB6F1-+/+ mice. Nevertheless, ethanol produced detectable, and in some cases substantial, gastric injury even in the complete absence of mast cells. Thus, ethanol can produce some damage to the gastric mucosa independently of mast cells. But these data suggest that under certain circumstances mast cells can augment the area of acute gastric injury induced by ethanol.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aures D., Håkanson R., Schauer A. Histidine decarboxylase and DOPA decarboxylase in the rat stomach. Properties and cellular localization. Eur J Pharmacol. 1968 Jun;3(3):217–234. doi: 10.1016/0014-2999(68)90135-0. [DOI] [PubMed] [Google Scholar]
- Barker J. E., Bernstein S. E. Hertwig's anemia: characterization of the stem cell defect. Blood. 1983 Apr;61(4):765–769. [PubMed] [Google Scholar]
- Cho C. H., Hung K. M., Ogle C. W. The aetiology of gastric ulceration induced by electrical vagal stimulation in rats. Eur J Pharmacol. 1985 Apr 2;110(2):211–217. doi: 10.1016/0014-2999(85)90213-4. [DOI] [PubMed] [Google Scholar]
- Crowle P. K., Reed N. D. Bone marrow origin of mucosal mast cells. Int Arch Allergy Appl Immunol. 1984;73(3):242–247. doi: 10.1159/000233476. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Simpson B. A., Richerson H. B., Leskowitz S., Karnovsky M. J. Cutaneous basophil hypersensitivity. II. A light and electron microscopic description. J Exp Med. 1970 Sep 1;132(3):558–582. doi: 10.1084/jem.132.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New approaches for the analysis of mast cell maturation, heterogeneity, and function. Fed Proc. 1987 Apr;46(5):1906–1914. [PubMed] [Google Scholar]
- Guth P. H., Code C. F. Histamine release and gastric mucosal damage. Gastroenterology. 1978 Mar;74(3):622–623. [PubMed] [Google Scholar]
- Guth P. H., Hall P. Microcirculatory and mast cell changes in restraint-induced gastric ulcer. Gastroenterology. 1966 Apr;50(4):562–570. [PubMed] [Google Scholar]
- HIATT R. B., KATZ L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962 May;37:541–545. [PubMed] [Google Scholar]
- Hansen D. G., Aures D., Grossman M. I. Histamine augments gastric ulceration produced by intravenous aspirin in cats. Gastroenterology. 1978 Mar;74(3):540–543. [PubMed] [Google Scholar]
- Howard E. B., Sawa T. R., Nielsen S. W., Kenyon A. J. Mastocytoma and gastroduodenal ulceration. Gastric and duodenal ulcers in dogs with mastocytoma. Pathol Vet. 1969;6(2):146–158. doi: 10.1177/030098586900600205. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Liedberg G. The role of endogenous gastrin in the activation of gastric histidine decarboxylase in the rat. Effect of antrectomy and vagal denervation. Eur J Pharmacol. 1970 Sep 1;12(1):94–103. doi: 10.1016/0014-2999(70)90033-6. [DOI] [PubMed] [Google Scholar]
- Johnson L. R. Source of the histamine released during damage to the gastric mucosa by acetic acid. Gastroenterology. 1968 Jan;54(1):8–15. [PubMed] [Google Scholar]
- Kitamura Y., Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979 Mar;53(3):492–497. [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Go S., Matsuda H., Hatanaka K., Seki M. Distribution of mast-cell precursors in hematopoeitic and lymphopoietic tissues of mice. J Exp Med. 1979 Sep 19;150(3):482–490. doi: 10.1084/jem.150.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Sonoda T., Nakano T., Hayashi C., Asai H. Differentiation processes of connective tissue mast cells in living mice. Int Arch Allergy Appl Immunol. 1985;77(1-2):144–150. doi: 10.1159/000233769. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Yokoyama M., Matsuda H., Shimada M. Coincidental development of forestomach papilloma and prepyloric ulcer in nontreated mutant mice of W/Wv and SI/SId genotypes. Cancer Res. 1980 Sep;40(9):3392–3397. [PubMed] [Google Scholar]
- Lacy E. R., Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982 Sep;83(3):619–625. [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Gravallese P. M., Stevens R. L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Galli S. J., Jackman R. W., Rosenberg R. D. Anticoagulantly active heparin-like molecules from mast cell-deficient mice. Am J Physiol. 1986 May;250(5 Pt 2):H879–H888. doi: 10.1152/ajpheart.1986.250.5.H879. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Kaliner M., Donlon M. A. The mast cell. Crit Rev Immunol. 1981 Sep;3(1):23–74. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle C. W., Lau H. K. Disodium cromoglycate: a novel gastric antiulcer agent? Eur J Pharmacol. 1979 May 15;55(4):411–415. doi: 10.1016/0014-2999(79)90117-1. [DOI] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H. R., Austen K. F., Stevens R. L., Galli S. J., Kitamura Y. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med. 1987 Mar 1;165(3):615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Rhodes J., Wheeler M. H., Meek E. M., Newcombe R. G. The role of histamine receptors in the pathophysiology of gastric mucosal damage. Gastroenterology. 1977 Jan;72(1):67–71. [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- SELYE H., JEAN P., CANTIN M. Prevention by stress and cortisol of gastric ulcers normally produced by 48/80. Proc Soc Exp Biol Med. 1960 Feb;103:444–446. doi: 10.3181/00379727-103-25552. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Shimada M., Kitamura Y., Yokoyama M., Miyano Y., Maeyama K., Yamatodani A., Takahashi Y., Tatsuta M. Spontaneous stomach ulcer in genetically mast-cell depleted W/Wv mice. Nature. 1980 Feb 14;283(5748):662–664. doi: 10.1038/283662a0. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Lewin K. J., Beaven M. A. Isolation of histamine-containing cells from rat gastric mucosa: biochemical and morphologic differences from mast cells. Gastroenterology. 1981 Apr;80(4):717–727. [PubMed] [Google Scholar]
- Szabo S., Trier J. S., Brown A., Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985 Jan;88(1 Pt 2):228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- Szabo S., Trier J. S., Brown A., Schnoor J., Homan H. D., Bradford J. C. A quantitative method for assessing the extent of experimental gastric erosions and ulcers. J Pharmacol Methods. 1985 Feb;13(1):59–66. doi: 10.1016/0160-5402(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Thunberg R. Localization of cells containing and forming histamine in the gastric mucosa of the rat. Exp Cell Res. 1967 Aug;47(1):108–115. doi: 10.1016/0014-4827(67)90214-5. [DOI] [PubMed] [Google Scholar]
- Yamatodani A., Maeyama K., Watanabe T., Wada H., Kitamura Y. Tissue distribution of histamine in a mutant mouse deficient in mast cells: clear evidence for the presence of non-mast-cell histamine. Biochem Pharmacol. 1982 Feb 1;31(3):305–309. doi: 10.1016/0006-2952(82)90175-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Tomoi M., Taguchi T., Nakano T., Asai H., Ono T., Kitamura Y. Fatal antral ulcer in conventionally fed W/Wv mutant mice given indomethacin by injection. Am J Pathol. 1985 Jun;119(3):367–375. [PMC free article] [PubMed] [Google Scholar]