Abstract
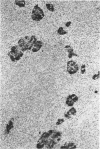
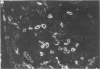
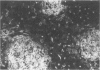
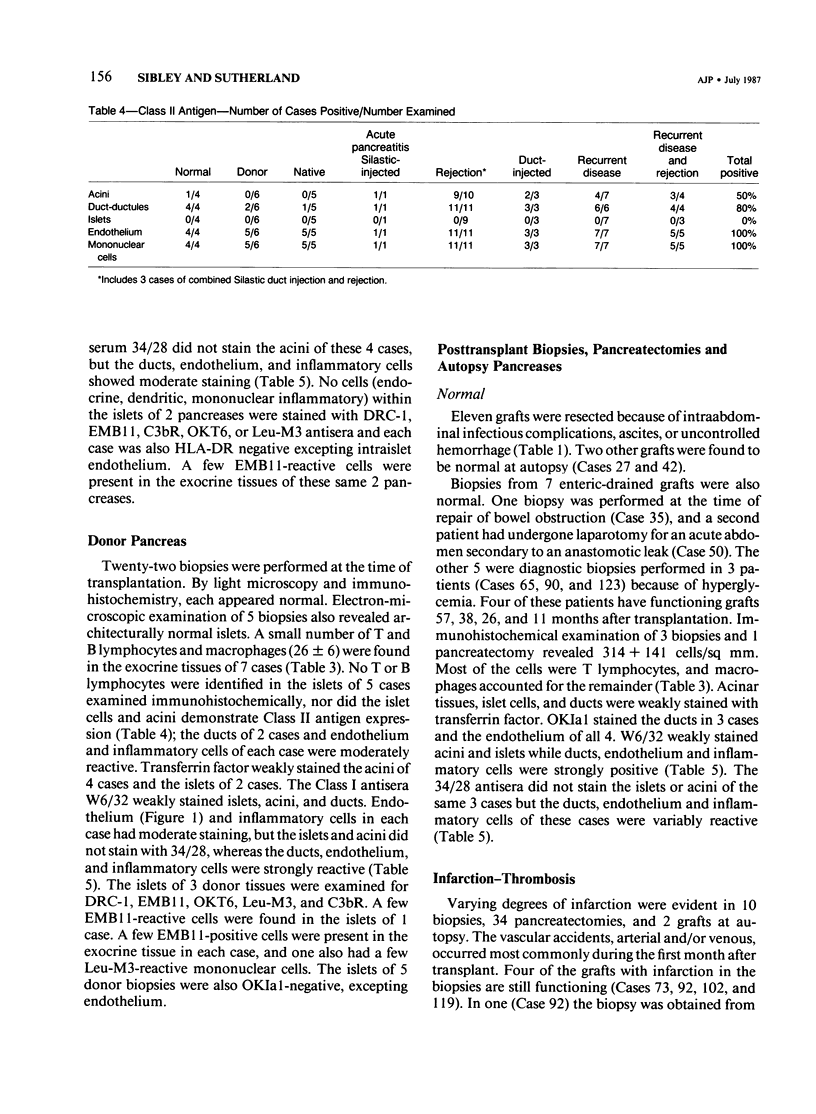
The authors examined tissues obtained by biopsy, pancreatectomy, and autopsy from 100 pancreas grafts to determine the cause of dysfunction or failure of the graft. Immunohistologic examination of 42 tissues to determine the mononuclear cell phenotypes and Class I and II antigen expression was performed as well. Technical factors--infections, thrombosis, obstruction--accounted for a large number of graft losses, but immunologic-mediated mechanisms resulted in graft dysfunction and failure as well. Pleomorphic inflammatory infiltrates were present in grafts with acute rejection, as well as Silastic and Prolamine duct-obstructed grafts. Criteria useful in the identification of acute rejection from pancreatitis included a more intense, predominantly mononuclear cell infiltration of transformed lymphocytes in the exocrine pancreas and evidence of vascular rejection--endovasculitis or fibrinoid necrosis. Increased expression and/or induction of Class I and II antigens on pancreatic constituents occurred in grafts with evidence of acute rejection, but also with Silastic and prolamine duct-obstructed pancreatitis. An isletitis occurred in 25% of the grafts. Nine of the 25 grafts (36%) with isletitis also had selective loss of beta cells from the islets. Recurrent diabetes mellitus appeared to have developed in these cases, which accounted for loss of graft function.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnes S., Castagneto M., Castiglioni G. C. Segmental pancreatic transplantation in the pig: comparative study of different techniques. Transplant Proc. 1980 Dec;12(4 Suppl 2):129–134. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Baumgartner D., Largiadèr F. Simultaneous renal and intraperitoneal segmental pancreatic transplantation: the Zürich experience. World J Surg. 1984 Apr;8(2):267–269. doi: 10.1007/BF01655146. [DOI] [PubMed] [Google Scholar]
- Blanc-Brunat N., Dubernard J. M., Touraine J. L., Neyra P., Dubois P., Paulin C., Traeger J. Pathology of the pancreas after intraductal neoprene injection in dogs and diabetic patients treated by pancreatic transplantation. Diabetologia. 1983 Aug;25(2):97–107. doi: 10.1007/BF00250896. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Du Toit D. F., Reece-Smith H., McShane P., Denton T., Morris P. J. Prolongation of segmental pancreatic allografts in dogs receiving cyclosporin A. Transplantation. 1982 Apr;33(4):432–437. [PubMed] [Google Scholar]
- Dubernard J. M., Traeger J., Bosi E., Gelet A., Yafi S. E., Devonec M., Piatti P. M., Chiesa R., Martin X., Mongin-Long D. Transplantation for the treatment of insulin-dependent diabetes: clinical experience with polymer-obstructed pancreatic grafts using neoprene. World J Surg. 1984 Apr;8(2):262–266. doi: 10.1007/BF01655145. [DOI] [PubMed] [Google Scholar]
- Dubernard J. M., Traeger J., Neyra P., Touraine J. L., Tranchant D., Blanc-Brunat N. A new method of preparation of segmental pancreatic grafts for transplantation: trials in dogs and in man. Surgery. 1978 Nov;84(5):633–639. [PubMed] [Google Scholar]
- Dubernard J. M., Traeger J., Touraine J. L., Bétuel H., Malik M. C. Rejection of human pancreatic allografts. Transplant Proc. 1980 Dec;12(4 Suppl 2):103–106. [PubMed] [Google Scholar]
- Faustman D., Hauptfeld V., Lacy P., Davie J. Prolongation of murine islet allograft survival by pretreatment of islets with antibody directed to Ia determinants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5156–5159. doi: 10.1073/pnas.78.8.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucar E., Mukai K., Foucar K., Sutherland D. E., Van Buren C. T. Colon ulceration in lethal cytomegalovirus infection. Am J Clin Pathol. 1981 Dec;76(6):788–801. doi: 10.1093/ajcp/76.6.788. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986 Nov;35(11):1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Gerdes J., Naiem M., Mason D. Y., Stein H. Human complement (C3b) receptors defined by a mouse monoclonal antibody. Immunology. 1982 Apr;45(4):645–653. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Stein H., Mason D. Y., Ziegler A. Human dendritic reticulum cells of lymphoid follicles: their antigenic profile and their identification as multinucleated giant cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(2):161–172. doi: 10.1007/BF02890379. [DOI] [PubMed] [Google Scholar]
- Gliedman M. L., Gold M., Whittaker J., Rifkin H., Soberman R., Freed S., Tellis V., Veith F. J. Pancreatic duct to ureter anastomosis for exocrine drainage in pancreatic transplantation. Am J Surg. 1973 Feb;125(2):245–252. doi: 10.1016/0002-9610(73)90035-4. [DOI] [PubMed] [Google Scholar]
- Gokel J. M. Simultaneous pancreas and kidney transplantation in man--graft morphology. Horm Metab Res Suppl. 1983;(13):76–77. [PubMed] [Google Scholar]
- Groth C. G., Lundgren G., Arner P., Collste H., Hårdstedt C., Lewander R., Ostman J. Rejection of isolated pancreatic allografts in patients with with diabetes. Surg Gynecol Obstet. 1976 Dec;143(6):933–940. [PubMed] [Google Scholar]
- Groth C. G., Lundgren G., Ostman J., Gunnarsson R. Experience with nine segmental pancreatic transplantations in preuremic diabetic patients in Stockholm. Transplant Proc. 1980 Dec;12(4 Suppl 2):68–71. [PubMed] [Google Scholar]
- Hancock W. W., Atkins R. C. Immunohistologic analysis of the cell surface antigens of human dendritic cells using monoclonal antibodies. Transplant Proc. 1984 Aug;16(4):963–967. [PubMed] [Google Scholar]
- Hart D. N., Newton M. R., Reece-Smith H., Fabre J. W., Morris P. J. Major histocompatibility complex antigens in the rat pancreas, isolated pancreatic islets, thyroid, and adrenal. Transplantation. 1983 Oct;36(4):431–435. doi: 10.1097/00007890-198310000-00015. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Braathen L. R., Thorsby E. Antigen presentation by vascular endothelial cells and epidermal Langerhans cells: the role of HLA-DR. Immunol Rev. 1982;66:57–77. doi: 10.1111/j.1600-065x.1982.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Bommer G., Commandeur G., Heitz P. The endocrine pancreas in chronic pancreatitis. Immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1978 Feb 10;377(2):157–174. doi: 10.1007/BF00427003. [DOI] [PubMed] [Google Scholar]
- Kyriakides G. K., Sutherland D. E., Olson L., Miller J., Najarian J. S. Segmental pancreatic transplantation in dogs. Transplant Proc. 1979 Mar;11(1):530–532. [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M. Transplantation of pancreatic islets. Annu Rev Immunol. 1984;2:183–198. doi: 10.1146/annurev.iy.02.040184.001151. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Simeonovic C. J., Warren H. S. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- Land W., Gebhardt C., Gall F. P., Weitz H., Gokel M. J., Stolte M. Pancreatic duct obstruction with prolamine solution. Transplant Proc. 1980 Dec;12(4 Suppl 2):72–75. [PubMed] [Google Scholar]
- Lau H., Reemtsma K., Hardy M. A. Prolongation of rat islet allograft survival by direct ultraviolet irradiation of the graft. Science. 1984 Feb 10;223(4636):607–609. doi: 10.1126/science.6420888. [DOI] [PubMed] [Google Scholar]
- Lillehei R. C., Simmons R. L., Najarian J. S., Weil R., Uchida H., Ruiz J. O., Kjellstrand C. M., Goetz F. C. Pancreatico-duodenal allotransplantation: experimental and clinical experience. Ann Surg. 1970 Sep;172(3):405–436. doi: 10.1097/00000658-197009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey N. J., Nolan M. S., Slater D. N., Savas C. P., Boyle P. F., Herold A., Beck S., Fox M. Rat pancreas allotransplantation: a short term comparison of rejection patterns with different methods of exocrine drainage. J Pathol. 1983 Mar;139(3):259–274. doi: 10.1002/path.1711390304. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Nerup J., Dinarello C. A., Svenson M., Nielsen J. H. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986 Jan;29(1):63–67. doi: 10.1007/BF02427283. [DOI] [PubMed] [Google Scholar]
- Morrow C. E., Sutherland D. E., Steffes M. W., Najarian J. S., Bach F. H. H-2 antigen class: effect on mouse islet allograft rejection. Science. 1983 Mar 18;219(4590):1337–1339. doi: 10.1126/science.6402817. [DOI] [PubMed] [Google Scholar]
- Munda R., Alexander J. W., First M. R., Knowles H. C., Weiss M. A. Synchronous transplantation of a kidney and duct-obliterated segmental pancreas: report of a case. Transplant Proc. 1980 Dec;12(4 Suppl 2):98–102. [PubMed] [Google Scholar]
- Munda R., First M. R., Webb C., Alexander J. W. Clinical experience with segmental pancreatic allografts. Transplant Proc. 1984 Jun;16(3):692–694. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Collins T., Cotran R. S., Ault K. A., Fiers W., Krensky A. M., Clayberger C., Reiss C. S., Burakoff S. J. Interactions of T lymphocytes with human vascular endothelial cells: role of endothelial cells surface antigens. Immunobiology. 1984 Dec;168(3-5):483–494. doi: 10.1016/s0171-2985(84)80132-1. [DOI] [PubMed] [Google Scholar]
- Shah K. H., Bitter-Suermann H., Save-Soderbergh J. Morphological findings in duct-ligated pancreas grafts in the rat. An analysis of isografts, allografts, and long-standing allografts in hosts conditioned by previous spleen allograft. Transplantation. 1980 Aug;30(2):83–89. [PubMed] [Google Scholar]
- Shienvold F. L., Alejandro R., Mintz D. H. Identification of Ia-bearing cells in rat, dog, pig, and human islets of Langerhans. Transplantation. 1986 Mar;41(3):364–372. doi: 10.1097/00007890-198603000-00016. [DOI] [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E., Goetz F., Michael A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985 Aug;53(2):132–144. [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Altered distribution of class I and class II MHC antigens during acute pancreas allograft rejection in the rat. Transplantation. 1985 Sep;40(3):234–239. doi: 10.1097/00007890-198509000-00002. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Najarian J. S. Intraperitoneal transplantation of immediately vascularized segmental pancreatic grafts without duct ligation. A clinical trial. Transplantation. 1979 Dec;28(6):485–491. [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Najarian J. S. One hundred pancreas transplants at a single institution. Ann Surg. 1984 Oct;200(4):414–440. doi: 10.1097/00000658-198410000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Rynasiewicz J. J., Baumgartner D., White D. C., Elick B. A., Najarian J. S. Segmental pancreas transplantation from living related and cadaver donors: a clinical experience. Surgery. 1981 Aug;90(2):159–169. [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger O., Herrmann S. H., Mescher M. F., Benacerraf B., Burakoff S. J. Cellular interactions in the generation of cytolytic T lymphocyte responses: role of Ia-positive splenic adherent cells in presentation in H-2 antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6091–6095. doi: 10.1073/pnas.77.10.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weringer E. J., Like A. A. Immune attack on pancreatic islet transplants in the spontaneously diabetic BioBreeding/Worcester (BB/W) rat is not MHC restricted. J Immunol. 1985 Apr;134(4):2383–2386. [PubMed] [Google Scholar]