Abstract
Some leguminous species of the genus Aeschynomene are specifically stem-nodulated by photosynthetic bradyrhizobia. To study the effect of bacterial photosynthesis during symbiosis, we generated a photosynthesis-negative mutant of the Bradyrhizobium sp. strain ORS278 symbiont of Aeschynomene sensitiva. The presence of a functional photosynthetic unit in bacteroids and the high expression of the photosynthetic genes observed in stem nodules demonstrate that the bacteria are photosynthetically active during stem symbiosis. Stem inoculation by the photosynthetic mutant gave a 50% decrease in stem-nodule number, which reduced nitrogen fixation activity and plant growth in the same proportion. These results indicate an important role of bacterial photosynthesis in the efficiency of stem nodulation.
Rhizobia interact symbiotically with leguminous plants by inducing nitrogen-fixing nodules on the roots. Some plants of the genus Aeschynomene, encountered in waterlogged soils or riverbanks, have the peculiar property of forming stem nodules. This very unusual behavior among leguminous plants is shared only with a few species of the genera Sesbania, Neptunia, and Discolobium (1). Rhizobia isolated from Aeschynomene stem nodules exhibit a property uncommon among other rhizobia of developing a photosynthetic system (2, 3). This was first demonstrated in the case of a strain isolated from Aeschynomene indica stem nodules and designated BTAi 1 (2). In ex planta culture, BTAi 1 required a photoperiod for production and accumulation of bacteriochlorophyll a and exhibited a photoheterotrophic and strictly aerobic photosynthesis (4, 5). In this respect, the photosynthetic activity of the bacteriochlorophyll-containing rhizobia most closely resembles that of the aerobic anoxygenic photosynthetic bacteria (6, 7).
Since the discovery of BTAi 1, several other rhizobia isolated from Aeschynomene stem nodules have been reported to produce bacteriochlorophyll a (8–13). These photosynthetic Aeschynomene rhizobia were shown to form a separate subbranch of the Bradyrhizobium rRNA lineage, distinct from Bradyrhizobium japonicum and Bradyrhizobium elkanii (13). All photosynthetic strains were shown to nodulate stem-nodulating Aeschynomene species specifically (13), indicating that the photosynthetic character of these rhizobia could play an important role during this unusual symbiosis. The demonstration by Evans et al. (4) that illumination of the stem nodules of A. indica by far-red light consistently accelerated acetylene reduction and the report of Fleischman and Kramer (3) on the presence of bacteriochlorophyll a in bacteroids isolated from stem nodules of Aeschynomene aspera provide further evidence for the importance of the photochemical activity of the endophytes in the stem nodules. However, no genetic study has determined the real contribution of the bacterial photosynthesis in the symbiotic interaction and its impact on plant growth.
In purple bacteria, most of the genes required for the formation of the photosystem are clustered in a 45-kb region (14). These include bch and crt genes coding respectively for the enzymes of the bacteriochlorophyll and carotenoid pathways and the puf genes coding for the subunits of the light-harvesting complex (pufB and pufA) and of the reaction center (RC) complex (pufL and pufM).
We report here the isolation and characterization of the puf genes from a photosynthetic Bradyrhizobium, strain ORS278, and reveal the unusual presence of a heme oxygenase gene (hmuO) just downstream from the pufM gene. ORS278 mutants deleted for heme oxygenase (HmuO) and RC subunit genes were constructed to elucidate the putative role of their products during symbiosis with Aeschynomene sensitiva.
Materials and Methods
Bacterial Strains and Growth Conditions.
Bradyrhizobium sp. strain ORS278 (12), called the wild-type strain throughout this study, and isogenic mutants 278ΔpufLM and 278ΔhmuO constructed in this work were grown in a modified YM medium (pH 6.8) containing (per liter) 2.5 g of sodium glutamate, 2 g of yeast extract, 0.66 g of K2HPO4, 0.05 g of NaCl, 0.1 g of MgSO4⋅7H2O, 0.04 g of CaCl2, and 0.004 g of FeCl3. Strains were grown aerobically in a gyratory shaker (170 rpm) at 37°C in either complete darkness or continuous white light with a bank of incandescent bulbs or under a light/dark cycle (16 h/8 h). Standard methods were used for Escherichia coli growth in Luria–Bertani (LB) medium supplemented with the appropriate antibiotics.
DNA Manipulations.
Unless otherwise stated, standard methods for DNA manipulation were used (15). To amplify the pufL and pufM genes of ORS278 by PCR, degenerate primers were used: pufLf, (5′-TTYTAYGTNGGNTTYTTYGG-3′) and pufMr, (5′-CCCATNGTCCANCGCCARAA-3′). PCR amplification, sequence reactions, and sequence analysis were performed as previously described (16).
Construction and Screening of a Genomic Library of Bradyrhizobium sp. Strain ORS278.
A genomic library of Bradyrhizobium ORS278 was constructed by using the SuperCos I cosmid vector kit (Stratagene). A positive clone, pSTM1, was found by PCR screening using the primers pufL.278f (5′-CACCCATCTCGATTGGGTGTCG-3′) and pufM.278r (5′-CTCCAGCTGCCCATGAAGATCG-3′).
Mapping of puf and hmuO Transcriptional Start Sites.
RNA was prepared as described by Cabanes et al. (17). The transcriptional start sites of the puf operon and the hmuO gene were mapped by primer extension as described by Batut et al. (18), using, respectively, the primer pufB.rev (5′-TATGGAATTCCCTAGCCTCCGATTC-3′) and the primer hmuO.rev (5′-GCCGTTTCGACGTGATTGGAATC-3′).
Construction of puf and hmuO Mutant Strains.
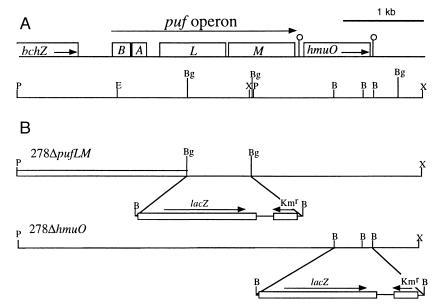
For the construction of a puf mutant, a 2.8-kb EcoRI–BamHI insert of pSTM1 containing the pufL and pufM genes of ORS278 was cloned into the EcoRI/BamHI sites of pUC18 (Roche, Meylan, France), liberated by EcoRI/HindIII digestion, and cloned into the EcoRI/HindIII sites of pBluescript (SK+) (Stratagene). Subsequently, the 0.8-kb BglII fragment containing a part of the pufL and pufM genes was replaced by the 4.7-kb BamHI lacZ-Kmr cassette of pKOK5 (19). The resulting 6.7-kb BamHI inserts containing the mutated pufL and pufM genes were cloned into the pJQ200mp18 suicide vector (20). To mutate the hmuO gene, the 1.8-kb BglII fragment of pSTM1 containing the hmuO gene of ORS278 was cloned into the BamHI sites of pJQ200mp18. The 3′ region of the hmuO gene was then deleted by BamHI digestion and replaced by the 4.7-kb BamHI lacZ-Kmr cassette of pKOK5. The pJQ200 derivatives obtained, which encoded a counterselectable sacB marker, were transformed into E. coli S17-1 (21) for mobilization into ORS278. After conjugation, colonies grown on YM medium containing kanamycin and nalidixic acid were plated onto YM medium containing 5% sucrose and kanamycin. Sucrose-resistant colonies were screened by PCR to verify that the double-crossover event had occurred. The genomic structure of the mutants was further verified by appropriate Southern blot hybridization of chromosomal DNA.
Preparation of Membranes.
Bradyrhizobium cells, resuspended in 50 mM Tris⋅HCl (pH 8), were disrupted by three passages through a French Press at 50 MPa. The suspension was spun for 20 min at 4,000 × g to remove the unbroken cells. The supernatant was then spun at 255,000 × g for 90 min. The pellet, resuspended in 50 mM Tris⋅HCl (pH 8), constituted the intracytoplasmic membrane fraction.
Preparation of Endosymbiotic Bacteria.
Nodules (5 g) were harvested 5 weeks after inoculation, resuspended with 10 ml of 50 mM KH2PO4/Na2PO4 buffer (pH 7.4) containing 1 g of polyvinylpyrrolidone, and ground with a mortar and pestle. Debris was removed by centrifugation for 5 min at 1,500 × g. Bacteroids were pelleted by centrifugation of the supernatant for 8 min at 8,000 × g, washed twice with 50 mM phosphate buffer (pH 7.4) containing 2 mM MgSO4 and 0.3 M sucrose, and resuspended in 1 ml of 50 mM phosphate buffer (pH 7.4).
Absorption and Light-Induced Absorption Change Measurements.
Light-induced absorption changes on intact cells were performed as previously described (22). Absorption spectra of bacteroids or membranes isolated from ex planta culture of ORS278 were recorded with a Cary 50 probe spectrophotometer.
Plant Tests.
A. sensitiva seeds were surface-sterilized with concentrated sulfuric acid for 40 min, abundantly rinsed with sterile water, allowed to soak overnight in water, and transferred to plastic pots (8-cm diameter) filled with atapulgite. The plants were grown in a greenhouse at 27–36°C with a relative humidity of 70–80%. Plants were watered regularly and supplied once a week with a plant nutrition solution (23) supplemented with 1 mM KNO3 until inoculation. After four weeks, the plants were root or stem inoculated. For root inoculation tests, 1 ml of a culture was deposited at the bottom of the plant, whereas stem inoculation was performed by careful painting. The inocula were prepared by growing a culture of either the wild-type strain or the mutant bacterial strains for 3 days, centrifuging the cells, washing them twice and resuspending them in water to obtain an OD of 0.5 at 600 nm. The plants were harvested 5 weeks after inoculation. Nitrogen-fixing activity was estimated on the entire plant by measurement of acetylene reducing activity according to Hardy et al. (24), and the dry mass was measured.
β-Galactosidase Assays and Light Microscopy.
Forty-micrometer-thick sections of fresh nodules were made by using a Leica VT1000S Vibratome and histochemically treated for β-galactosidase staining as described by Boivin et al. (25).
Results
Isolation and Characterization of the puf Operon of Bradyrhizobium Sp. Strain ORS278.
PCR amplification using degenerate primers pufLf/pufMr, based on conserved domains of the PufL and PufM proteins (data not shown), gave, with the genomic DNA of the ORS278 strain as template, a fragment of 1,546 bp (probe A), whose sequence was highly similar to those of the pufL and pufM genes from other photosynthetic bacteria. Subsequently, two specific primers (pufL278f/pufM278r), based on the sequence of the amplified fragment, were designed and used for PCR screening of a genomic DNA library of the ORS278 strain. A positive clone, pSTM1, which contains an insert of approximately 35 kb, was used to characterize the puf gene cluster. A 5.1-kb region in the DNA insert of the pSTM1 cosmid, showing positive hybridization to probe A, was sequenced and analyzed, as shown in Fig. 1. This nucleotide sequence had five ORFs, each of which had a putative Shine–Dalgarno box preceding the start codon, ATG. Comparisons with the puf genes of other photosynthetic bacteria revealed that four of the five ORFs were pufB, pufA, pufL, and pufM, which encode the β and α subunits of the LH1 light-harvesting complex, and the L and M subunits of the RC complex, respectively. PufL and PufM proteins of Bradyrhizobium sp. strain ORS278 showed the greatest identity to those of Rhodopseudomonas palustris (75% and 69%, respectively) and Rhodospirillum rubrum (68% and 69%, respectively).
Figure 1.
Physical map of the Bradyrhizobium sp. strain ORS278 puf region and genetic structure of puf and hmuO mutations. (A) The genes are indicated by open boxes. B, pufB; A, pufA; L, pufL; M, pufM. The positions of putative hairpin structures are indicated by open circles. The positions of relevant restriction sites are also shown. P, PstI; X, XhoI; E, EcoRI; B, BamHI; Bg, BglII. (B) The structures of puf and hmuO mutations are shown along with the corresponding strain names. Horizontal arrows indicate the orientation of the cassette containing the reporter gene lacZ and Kmr.
The region upstream of the pufB gene of Bradyrhizobium sp. strain ORS278 contained a nucleotide sequence showing significant sequence identity (73%) with the 3′-region of bchZ of R. rubrum, which coded for an enzyme involved in the conversion of chlorophyll to bacteriochlorophyll (26). Downstream of the pufM gene, another ORF encoding a putative heme oxygenase was identified unexpectedly. The use of a hmuO-specific probe demonstrated that ORS278 possesses a unique copy of the hmuO gene (data not shown). The enzyme encoded by this gene is highly conserved throughout the animal and plant kingdoms. It catalyzes the cleavage of the heme ring with the production of biliverdin, Fe3+, and carbon monoxide (27, 28). The putative HmuO protein contained 203 amino acids and showed homology with the corresponding genes from a variety of organisms: 36% with Synechocystis, 36% with the human HO-1, 35% with Rhodella violacea, and 30% with Corynebacterium diphtheriae. Taking into account conservative substitutions of amino acids, similarities reached 49%, 49%, 46%, and 45%, respectively.
The pufM and hmuO genes are closely linked physically—i.e., the supposed ATG start codon of hmuO was located 94 bp after the pufM stop codon. This could indicate that the hmuO gene belongs to the puf operon. However, a putative hairpin loop structure was detected between these two genes. This hairpin was characterized by a stem of 11 bp adjacent to 4 T residues, and indicating a possible transcriptional termination signal after the pufM gene. An additional putative hairpin loop structure (stem of 8 bp adjacent to 3 T residues) was identified downstream from the hmuO gene, suggesting that the hmuO gene corresponds to a single transcript.
Mapping of the puf and hmuO Start Sites.
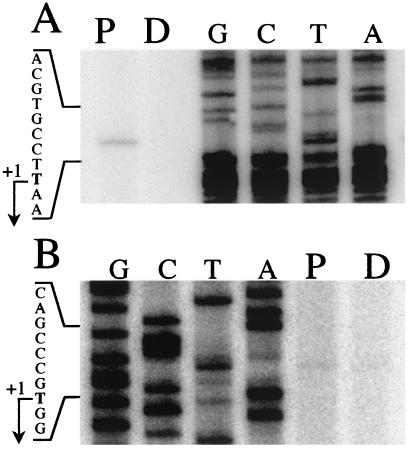
Primer extension analysis had a twofold objective: (i) to identify the precise 5′ end of the puf operon and (ii) to determine whether the hmuO gene belongs to another transcription unit. The 32P-labeled primers (pufB.rev and hmuO.rev, see Materials and Methods) were hybridized to total RNAs isolated from cells grown under either continuous dark (D) or a dark–light cycle (P). For the puf operon start site analyses (Fig. 2A), only the dark–light cycle culture condition gave one single transcriptional initiation site, which corresponded to a T on the RNA level located 111 bp upstream from the putative translational start of pufB. Results obtained with the hmuO.rev primer showed the presence of one 5′ end of hmuO mRNA corresponding to a T located 153 bp upstream from the putative translational start of the hmuO gene (Fig. 2B).
Figure 2.
Determination of the transcriptional start sites of the puf operon (A) and hmuO (B). Primer extension analyses were performed with RNAs extracted from ORS278 strain cultured under a dark–light illumination cycle (P) or in darkness (D). As a reference, a DNA sequencing ladder is shown (lanes G, C, T, and A). The sequences were obtained with the same primers used for the primer extensions. The transcription start points (+1) are indicated by arrows.
Photosynthetic Activity in Bradyrhizobium Sp. Strain ORS278 and in puf and hmuO Mutants.
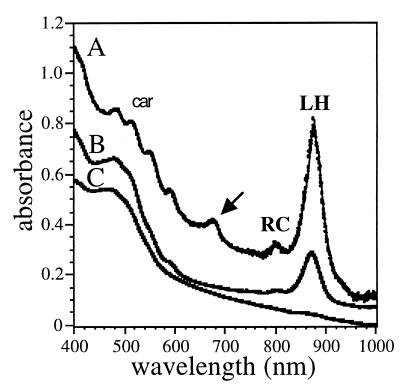
Fig. 3 shows a comparison of the absorption spectra of membranes isolated from the wild-type strain grown under cyclic illumination ex planta and from membranes isolated from A. sensitiva stem nodule endophytes. Both types of membranes display absorption bands characteristic of the presence of light-harvesting complexes, RCs, and carotenoids. The near-infrared part of the spectrum is very similar to the one observed for Rhodospirillum rubrum membranes, demonstrating the absence of light-harvesting II complexes. The photosynthetic apparatus is expressed only by cells grown under a light–dark cycle (not shown), as has already been described for BTAi 1 (3).
Figure 3.
Visible and near-infrared absorption spectra of membranes of ORS278 and 278ΔpufLM strains. Line A corresponds to the spectrum of membranes of strain ORS278 isolated from stem nodules and line B corresponds to that of bacteria grown ex planta. Because of the high scattering properties of the bacteroid preparation, the optical density at 1000 nm has been arbitrary decreased from 1 to 0.1. Line C corresponds to the absorption of membranes isolated from strain 278ΔpufLM. The arrow indicates a small contamination by chloroplasts in the bacteroid preparation. Absorption of the RC, light-harvesting complexes (LH), and carotenoids (car) is also indicated.
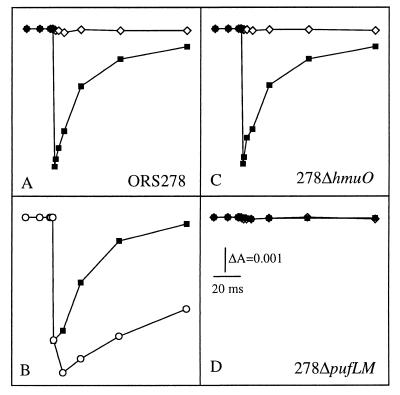
The photosynthetic activity of the bacteria was determined by monitoring light-induced absorption changes in intact cells. Fig. 4A shows the kinetics of the light-induced changes observed in cells grown under a light–dark cycle and placed under aerobic conditions at 422 nm, a wavelength characteristic of the redox state of the cytochromes. Under these conditions, a biphasic photo-oxidation of a cytochrome is observed with a fast phase unresolved (<50 μs) and a slow phase with a halftime of 200 μs (not shown), followed by its reduction in the 10-ms time range. The light-induced difference spectra, detected 50 μs and 1 ms after the actinic flash, confirm the photo-oxidation of a cytochrome (not shown). The addition of myxothiazol, a specific inhibitor of the bc1 complex, strongly slows the kinetics of rereduction of the photo-oxidized cytochrome (Fig. 4B). When the cells are placed under anaerobic conditions, no light-induced absorbance changes can be detected (Fig. 4A), in agreement with the aerobic character of the bacterium. Light-induced absorption changes have also been performed with intact cells to assay the photochemical competence of 278ΔpufLM and 278ΔhmuO, mutants deleted in the pufLM and hmuO genes, respectively (Fig. 1). As expected, no light-induced photo-oxidation of cytochrome can be detected upon illumination of 278ΔpufLM cells even under aerobic conditions (Fig. 4D). In addition, the deletion of the RC genes induces a profound modification of the absorption spectrum of the intracytoplasmic membrane (Fig. 3C). On the other hand, the photochemical activity and the absorption spectrum of the 278ΔhmuO mutant grown under cyclic illumination were found to be identical to the one observed for the wild-type strain (Fig. 4C and data not shown).
Figure 4.
The kinetics of cytochrome rereduction measured at 422 nm on whole cells of Bradyrhizobium. The cells were resuspended in fresh growth medium made either aerobic by bubbling air or anaerobic by bubbling argon through the suspensions. (A) Strain ORS278, aerobic (■) or anaerobic (◊) conditions. (B) Strain ORS278, aerobic condition in the absence (■) or presence (○) of 40 μM myxothiazol. (C and D) Strains 278ΔhmuO and 278ΔpufLM, aerobic (■) or anaerobic (⋄) conditions.
In Planta and ex Planta Expression of the puf and hmuO Genes.
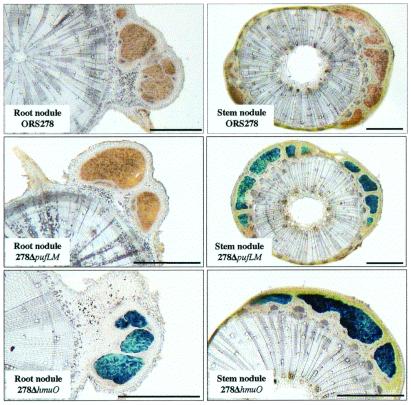
The expression of the puf and hmuO genes was assayed in planta by inoculation of A. sensitiva with the two mutants bearing the lacZ-hmuO and lacZ-puf fusions. Nodules were harvested 20 days after inoculation, and transverse sections were stained for β-galactosidase activity (Fig. 5). A strong β-galactosidase activity was visualized in the central tissues of both stem and root nodules with the lacZ-hmuO fusion. In contrast, the lacZ-puf fusion shows a high β-galactosidase activity exclusively in the stem nodules. These results indicate that both hmuO and puf genes are expressed during symbiosis but that the puf genes are specifically expressed in the stem nodules, in agreement with the observation of the photosynthetic unit exclusively in these nodules (Fig. 3). Ex planta, we observed that puf expression requires a minimum of 3% oxygen (data not shown), confirming the aerobic character of Bradyrhizobium sp. strain ORS278.
Figure 5.
Histochemical localization of β-galactosidase activity in stem and root nodules inoculated with ORS278, 278ΔpufLM, and 278ΔhmuO strains. (All magnification bars = 1 mm.)
Symbiotic Phenotypes of puf and hmuO Mutants.
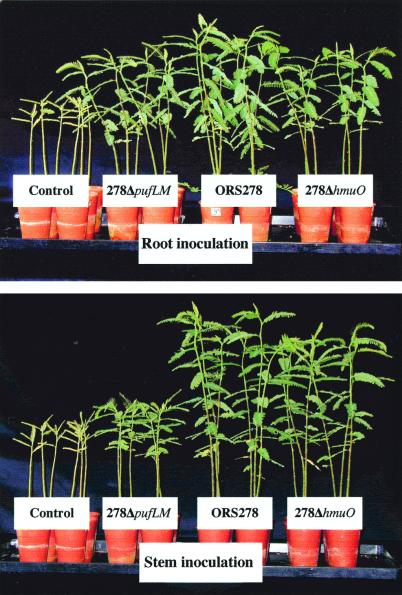
The two mutants, 278ΔpufLM and 278ΔhmuO, were used to investigate the requirement for bacterial photosynthesis and heme oxygenase on both root- and stem-nodulation, and nitrogen fixation during symbiosis. Root inoculation with both mutants, and stem inoculation with the 278ΔhmuO mutant, resulted in nodule number and plant dry weight similar to those inoculated with the wild-type strain (Fig. 6 and Table 1). The HmuO mutant induced stem nodulation and nitrogen fixation at a rate similar to that of the wild-type strain, suggesting that the HmuO activity is not an essential factor for symbiosis. In contrast, stem inoculation with the mutant 278ΔpufLM induced a lag of 3 days in nodulation (data not shown) and a drastic decrease (about 50%) in stem nodule number, plant growth, and nitrogenase activity (Table 1). These results, observed in three independent experiments, demonstrate that bacterial photosynthesis plays an important role in stem nodulation efficiency.
Figure 6.
Comparison of the growth of the plants of A. sensitiva, roots or stems inoculated with ORS278, 278ΔpufLM, and 278ΔhmuO strains. Photographs were taken 5 weeks after inoculation.
Table 1.
Symbiotic phenotypes of 278ΔpufLM and 278ΔhmuO mutants compared with those of the wild-type strain ORS278
| Strain | Root inoculation
|
Stem
inoculation
|
|||||
|---|---|---|---|---|---|---|---|
| No. of root nodules*/plant | Plant dry wt,† g/plant | Fixation activity,‡ μmol/h/plant | No. of stem nodules*/plant | Plant dry wt,† g/plant | Fixation activity,‡ μmol/h/plant | Fixation activity/nodule, μmol/h/nodule§ | |
| ORS278 | 33 ± 7 | 1.78 ± 0.3 | 63 ± 12 | 19 ± 3 | 1.94 ± 0.3 | 75 ± 11 | 4 ± 0.8 |
| 278ΔpufLM | 29 ± 9 | 1.47 ± 0.2 | 57 ± 10 | 9 ± 3 | 0.97 ± 0.2 | 34 ± 5 | 3.8 ± 0.7 |
| 278ΔhmuO | 32 ± 9 | 1.44 ± 0.3 | 51 ± 10 | 20 ± 4 | 1.62 ± 0.2 | 63 ± 11 | 3.3 ± 0.7 |
| No inoculum | 0 | 0.47 ± 0.1 | 0 | 0 | 0.47 ± 0.1 | 0 | — |
All values are the means ± standard errors of 10 individual plants.
The number of nodules was measured 15 days after inoculation.
The dry weight was measured 5 weeks after inoculation.
The acetylene reduction assay was performed 5 weeks after inoculation.
These values were obtained by dividing the values of the acetylene reduction activity of the entire plant by the number of nodules measured on the stem 15 days after inoculation.
It is noteworthy that no difference is observed in the stem nodule structures induced by the wild-type or mutant 278ΔpufLM (Fig. 5). Furthermore, the few nodules formed by the photosynthetic mutant exhibited the same individual nitrogenase activity as the wild-type nodules (Table 1). In addition, we observed that the expression of puf genes is already detected 5 days after inoculation, before the appearance of the nodules, specifically at the emergence of root primordia (data not shown). These data indicate that bacterial photosynthesis plays a role in an early step of infection rather than in the energy requirement for nitrogenase activity.
Discussion
Absorption spectroscopy and photochemical measurements clearly indicated the presence of a functional photosynthetic apparatus in intact cells of ORS278. The occurrence of a light-induced cyclic electron transfer involving electron transfer between the RC and cytochromes bc1 is attested by the fast photo-oxidation of a cytochrome c after its rapid rereduction, a reaction specifically inhibited by myxothiazol. These results show that a complete photoinduced electron transfer occurs in a photosynthetic Bradyrhizobium, in full agreement with several studies reported for the BTAi 1 strain (3). While typical photosynthetic bacteria can grow under continuous light and anaerobiosis, the synthesis of the photosynthetic apparatus of ORS278 occurs only under cyclic illumination, and its photosynthetic activity requires aerobic conditions. The inability of aerobic photosynthetic bacteria to grow at the expense of light under anaerobic conditions has been related to the reduction of the primary electron acceptor because of the more positive value of its midpoint potential compared with those of the purple photosynthetic bacteria (29–31). However, in a recent report, Scharze et al. (32) cast doubt on this interpretation, since they observed that the high value for the midpoint potential of the primary electron acceptor obtained for Roseobacter denitrificans (29) was due to lack of equilibration during the redox titration.
The organization of the puf operon of strain ORS278 is one of the simplest described so far because it contains only the pufB, pufA, pufL, and pufM genes. However, the region downstream of the puf operon surprisingly shows a putative ORF encoding a heme oxygenase. Start point analysis and lacZ fusion studies (data not shown) showed that the expression of the hmuO gene is controlled by its own promoter located 153 bp in front of the putative start codon. Nevertheless, we cannot completely exclude that the hmuO gene could also be partially transcribed from the puf operon. Two main functions of heme oxygenase have been proposed for the bacteria in which this gene has been identified recently. In Corynebacterium diphtheriae, HmuO is involved in the release of iron from hemoglobin (33), whereas in the cyanobacterium Synechocystis this enzyme is involved in the biosynthesis of linear tetrapyrroles that act as chromophores in phycobilins (34). We propose instead that the hmuO gene is involved, as in both cyanobacteria and plants, in the chromophore biosynthesis of a regulatory receptor. Our proposal is based on the localization of this gene near the photosynthetic gene cluster and the assumptions that light exerts its regulatory influence on bacteriochlorophyll accumulation in BTAi 1 via photoreceptors (3, 5).
Phylogenetic analysis of pufL and pufM sequences available from photosynthetic α-proteobacteria (data not shown) shows that the puf genes of strain ORS278 are closely related to those of the anaerobic photosynthetic bacterium Rhodopseudomonas palustris (supported by a bootstrap value of 99%). Despite its functional similarity to aerobic photosynthetic bacteria, ORS278 is phylogenetically distant from Roseobacter denitrificans and Erythrobacter longus. A similar conclusion has also been deduced previously on the basis of a 16S rDNA analysis (35). The congruence of these two approaches indicates that the photosynthetic character of strain ORS278 is not the result of a lateral transfer, as suggested for other photosynthetic bacteria (36), but rather evolved from a photosynthetic ancestor common to R. palustris. Most bradyrhizobia are root symbionts living in an environment devoid of light. As a consequence, the photosynthetic system is no longer necessary and could have been progressively lost. In contrast, the maintenance of the photosynthetic function in the stem symbionts of Aeschynomene might be the result of a selective advantage. This last hypothesis is in full agreement with our demonstration that the disruption of the photosynthetic genes induced a 50% decrease in stem-nodule number with the photosynthesis-negative mutant compared with either the wild-type strain or the HmuO mutant used as controls. This reduction of stem nodulation led to a decrease in the nitrogen fixation activity, and thus, plant growth. This result clearly demonstrates that bacterial photosynthesis plays a significant role in the efficiency of stem infection. In the stem-nodulating legumes, rhizobial infection starts through “crack entry”—e.g., bacteria gain access to the cortical cells of the host plant via ruptures in the stem epidermis caused by the emergence of root primordia (37, 38). Sesbania rostrata, nodulated by the nonphotosynthetic Azorhizobium caulinodans (1), and Aeschynomene afraspera, nodulated by both photosynthetic and nonphotosynthetic bacteria (13), are characterized by the presence on their stems of numerous protruding root primordia. These primordia pierce the epidermal layer and form at their base a large annular cavity (37, 38) where the bacteria can easily multiply. Therefore, the bacteria are protected from the outside environment and gain energy substrates from either cell debris or exudates. In contrast, in Aeschynomene sensitiva, most root primordia remain embedded beneath a few layers of epidermal cells and are rarely accessible to the bacteria (39). We suggest that, on the surface of the stem, under aerobic alternative light and dark conditions, the photosynthetic electron transfer chain contributes to an increase in the proton gradient for either ATP production or substrate transport. This energy either might allow the bacteria to survive on the stem where the availability of assimilable substrates is limited or be used by the bacteria to reach the cortical cells.
The high expression of the puf genes observed in the stem nodules and the detection of a functional photosynthetic unit in bacteroids indicate that the bacteria are photosynthetically active during stem symbiosis, as previously suggested (3, 4). We cannot exclude that this bacterial photosynthesis also plays an auxiliary role during the symbiosis by directly furnishing additional energy to the bacteroid. This might limit the demand for plant photosynthates and promote plant growth. The paradox of this last proposal is that the bacterial photosynthetic activity depends on aerobic conditions, whereas nitrogen fixation occurs only under microaerobic conditions. This implicates a fine regulation of the oxygen tension in the bacteroid, probably by means of leghemoglobin, to a narrow range where bacterial photosynthesis and nitrogenase activity can operate simultaneously.
Acknowledgments
We thank A. M. Garneronne for her technical assistance in the start site analysis and W. d'Haeze for his support in mutant construction. This work was supported in part by the Centre National de la Recherche Scientifique (Dynamique de la Biodiversité et Environnement).
Abbreviations
- RC
reaction center
- HmuO
heme oxygenase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF182374).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250484097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250484097
References
- 1.Boivin C, Ndoye I, Molouba F, de Lajudie P, Dupuy N, Dreyfus B. Crit Rev Plant Sci. 1997;16:1–30. [Google Scholar]
- 2.Eaglesham A R J, Ellis J M, Evans W R, Fleischman D E, Hungria M, Hardy R W F. In: Nitrogen Fixation: Achievements and Objectives. Gresshoff P M, Roth L E, Stacey G, Newton W L, editors. New York: Chapman and Hall; 1990. pp. 805–811. [Google Scholar]
- 3.Fleischman D, Kramer D. Biochim Biophys Acta. 1998;1364:17–36. doi: 10.1016/s0005-2728(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 4.Evans W R, Fleischman D E, Calvert H E, Pyati P V, Alter G M, Subra Rao N S. Appl Environ Microbiol. 1990;56:3445–3449. doi: 10.1128/aem.56.11.3445-3449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wettlaufer S H, Hardy R W F. Plant Cell Physiol. 1995;36:391–396. [Google Scholar]
- 6.Shimada K. In: Anoxygenic Photosynthetic Bacteria. Blankenship R E, Madigan M T, Bauer C E, editors. Dordrecht, The Netherlands: Kluwer; 1995. pp. 105–122. [Google Scholar]
- 7.Yurkov V V, Beatty J T. Microbiol Mol Biol Rev. 1998;62:695–724. doi: 10.1128/mmbr.62.3.695-724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladha J K, Pareek R P, So R, Becker M. In: Nitrogen Fixation: Achievements and Objectives. Gresshoff P M, Roth L E, Stacey G, Newton W L, editors. New York: Chapman and Hall; 1990. pp. 633–640. [Google Scholar]
- 9.Ladha J K, So R B. Int J Syst Bacteriol. 1994;44:62–73. doi: 10.1099/00207713-44-3-392. [DOI] [PubMed] [Google Scholar]
- 10.Wong F Y K, Stackebrandt E, Ladha J K, Fleischman D E, Date R A, Fuerst J A. Appl Environ Microbiol. 1994;60:940–946. doi: 10.1128/aem.60.3.940-946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Berkum P, Tully R E, Keister D L. Appl Environ Microbiol. 1995;61:623–629. doi: 10.1128/aem.61.2.623-629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorquin J, Molouba F, Dreyfus B L. Appl Environ Microbiol. 1997;63:1151–1154. doi: 10.1128/aem.63.3.1151-1154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molouba F, Lorquin J, Willems A, Hoste B, Giraud E, Dreyfus B, Gillis M, de Lajudie P, Masson-Boivin C. Appl Environ Microbiol. 1999;65:3084–3094. doi: 10.1128/aem.65.7.3084-3094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti M, Burke D H, Hearst J E. In: Anoxygenic Photosynthetic Bacteria. Blankenship R E, Madigan M T, Bauer C E, editors. Dordrecht, The Netherlands: Kluwer; 1995. pp. 775–805. [Google Scholar]
- 15.Sambrook J, Fristch E F, Maniatis T, Irwin N. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Hannibal L, Lorquin J, Angles d'Ortoli N, Garcia N, Chaintreuil C, Masson-Boivin C, Dreyfus B, Giraud E. J Bacteriol. 2000;182:3850–3853. doi: 10.1128/jb.182.13.3850-3853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabanes D, Boistard P, Batut J. Mol Plant-Microbe Interact. 2000;13:483–493. doi: 10.1094/MPMI.2000.13.5.483. [DOI] [PubMed] [Google Scholar]
- 18.Batut J, Daveran-Mingot M L, David M, Jacobs J, Garneronne A M, Kahn D. EMBO J. 1989;8:1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokotek W, Lotz W. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 20.Quandt J, Hynes M F. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 21.Simon R, Priefer U, Pühler A. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 22.Yurkov V, Schoepp B, Verméglio A. Photosynth Res. 1998;57:117–128. [Google Scholar]
- 23.Bertrand H, Plassard C, Pinochet X, Touraine B, Normand P, Cleyet-Marel J C. Can J Microbiol. 2000;46:229–236. doi: 10.1139/w99-137. [DOI] [PubMed] [Google Scholar]
- 24.Hardy R W F, Burn R C, Holten R D. Biochemistry. 1973;43:47–87. [Google Scholar]
- 25.Boivin C, Camut S, Malpica C A, Truchet G, Rosenberg C. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biel A J. In: Anoxygenic Photosynthetic Bacteria. Blankenship R E, Madigan M T, Bauer C E, editors. Dordrecht, The Netherlands: Kluwer; 1995. pp. 1125–1134. [Google Scholar]
- 27.Richaud C, Zabulon G. Proc Natl Acad Sci USA. 1997;94:11736–11741. doi: 10.1073/pnas.94.21.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poss K D, Tonegawa S. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamiya K, Iba K, Okamura K. Biochim Biophys Acta. 1987;890:127–133. [Google Scholar]
- 30.Yurkov V, Menin L, Schoepp B, Verméglio A. Photosynth Res. 1998;57:129–138. [Google Scholar]
- 31.Kramer D M, Kanazawa A, Fleishman D. FEBS Lett. 1997;417:275–278. doi: 10.1016/s0014-5793(97)01300-8. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze C, Carluccio A V, Venturoli G, Labahn A. Eur J Biochem. 2000;267:422–433. doi: 10.1046/j.1432-1327.2000.01018.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilks A, Schmitt M P. J Bacteriol. 1997;179:838–845. doi: 10.1128/jb.179.3.838-845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornejo J, Willows R D, Beale S I. Plant J. 1998;15:99–107. doi: 10.1046/j.1365-313x.1998.00186.x. [DOI] [PubMed] [Google Scholar]
- 35.Young J P W, Downer H L, Eardly B D. J Bacteriol. 1991;173:2271–2277. doi: 10.1128/jb.173.7.2271-2277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagashima K V P, Hiraishi A, Shimada K, Matsuura K. J Mol Evol. 1997;45:131–136. doi: 10.1007/pl00006212. [DOI] [PubMed] [Google Scholar]
- 37.Alazard D, Duhoux E. J Exp Bot. 1990;41:1199–1206. [Google Scholar]
- 38.Duhoux E. Can J Bot. 1984;62:982–994. [Google Scholar]
- 39.Alazard D, Duhoux E. J Plant Physiol. 1988;132:123–125. [Google Scholar]