Abstract
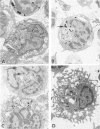
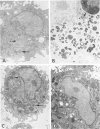
To determine whether the cytochemical localization of peroxidase activity could be used as a marker of monocyte influx into the lung during an inflammatory response, the authors studied the peroxidase phenotypes of lavaged alveolar macrophages from rats with bacille Calmette-Guérin (BCG)-induced pulmonary inflammation. Rats were immunized subcutaneously and 2 weeks later intravenously with BCG. During the early phase of pulmonary inflammation, an increase was observed in the numbers of alveolar macrophages with no peroxidase activity in the endoplasmic reticulum. These cells appeared to reflect monocyte influx into the injured lung. The later stages of inflammation were characterized by increased numbers of alveolar macrophages with peroxidase-positive endoplasmic reticulum, probably due to activation of enzymatic activity in situ. During the early phase, peroxidase activity was also observed within macrophage cytoplasmic inclusions, probably representing both primary monocyte lysosomes and internalized myeloperoxidase from inflammatory neutrophils. Serial observations indicated that the peroxidase-positive cytoplasmic inclusions became negative with time. It is concluded that inflammation-induced modulation of peroxidase activity in the endoplasmic reticulum and in cytoplasmic inclusions makes the alveolar macrophage peroxidase phenotype no more than a rough marker of monocyte influx into the inflamed lung.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beelen R. H., Broekhuis-Fluitsma D. M., Korn C., Hoefsmit C. M. Identification of exudate-resident macrophages on the basis of peroxidatic activity. J Reticuloendothel Soc. 1978 Feb;23(2):103–110. [PubMed] [Google Scholar]
- Beelen R. H., Fluitsma D. M. What is the relevance of exudate-resident macrophages? Immunobiology. 1982 Apr;161(3-4):266–273. doi: 10.1016/S0171-2985(82)80082-X. [DOI] [PubMed] [Google Scholar]
- Beelen R. H., Walker W. S. Dynamics of cytochemically distinct subpopulations of macrophages in elicited rat peritoneal exudates. Cell Immunol. 1983 Dec;82(2):246–257. doi: 10.1016/0008-8749(83)90159-4. [DOI] [PubMed] [Google Scholar]
- Beelen R. H., van't Veer M. B., Fluitsma D. M., Hoefsmit E. C. Identification of different peroxidatic activity patterns in human macrophages in vivo and in vitro. J Reticuloendothel Soc. 1978 Oct;24(4):351–362. [PubMed] [Google Scholar]
- Bentfeld M. E., Nichols B. A., Bainton D. F. Ultrastructural localization of peroxidase in leukocytes of rat bone marrow and blood. Anat Rec. 1977 Feb;187(2):219–240. doi: 10.1002/ar.1091870208. [DOI] [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Peroxidase activity of alveolar macrophages. Lab Invest. 1976 Jan;34(1):31–42. [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med. 1977 Feb 1;145(2):264–274. doi: 10.1084/jem.145.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Differences in peroxidase localization of rabbit peritoneal macrophages after surface adherence. Am J Pathol. 1978 Apr;91(1):107–117. [PMC free article] [PubMed] [Google Scholar]
- Bouwens L., Wisse E. Proliferation, kinetics, and fate of monocytes in rat liver during a zymosan-induced inflammation. J Leukoc Biol. 1985 May;37(5):531–543. doi: 10.1002/jlb.37.5.531. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Role of monocytes and interstitial cells in the generation of alveolar macrophages I. Kinetic studies of normal mice. Lab Invest. 1980 May;42(5):511–517. [PubMed] [Google Scholar]
- Breton-Gorius J., Guichard J., Vainchenker W., Vilde J. L. Ultrastructural and cytochemical changes induced by short and prolonged culture of human monocytes. J Reticuloendothel Soc. 1980 Mar;27(3):289–301. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Bakker J. M., De Wit A. W., Woelders H., Ginsel L. A., Daems W. T. On the origin of peritoneal resident macrophages. II. Recovery of the resident macrophage population in the peritoneal cavity and in the milky spots after peritoneal cell depletion. J Submicrosc Cytol. 1985 Apr;17(2):141–151. [PubMed] [Google Scholar]
- Deimann W., Seitz M., Gemsa D., Fahimi H. D. Endogenous peroxidase in the nuclear envelope and endoplasmic reticulum of human monocytes in vitro: association with arachidonic acid metabolism. Blood. 1984 Aug;64(2):491–498. [PubMed] [Google Scholar]
- Deimann W., Teckhaus L. Formation of a morphologically complex system by peroxidase-positive lysosomal elements in human monocytes. Blood. 1985 Sep;66(3):514–521. [PubMed] [Google Scholar]
- Dvorak A. M., Hammond M. E., Morgan E., Orenstein N. S., Galli S. J., Dvorak H. F. Evidence for a vesicular transport mechanism in guinea pig basophilic leukocytes. Lab Invest. 1980 Feb;42(2):263–276. [PubMed] [Google Scholar]
- Fahimi H. D. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970 Oct;47(1):247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Wong M., McIntyre D. Characterization of macrophage subpopulations responsive to activation by endotoxin and lymphokines. J Immunol. 1981 Jun;126(6):2474–2479. [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. A. Uptake and digestion of horseradish peroxidase in rabbit alveolar macrophages. Formation of a pathway connecting lysosomes to the cell surface. Lab Invest. 1982 Sep;47(3):235–246. [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Oka K., Miyazaki M., Kojima M. An electronmicroscopical study on peroxidase activity of rabbit monocytes, resident and exudate macrophages. Acta Pathol Jpn. 1982 May;32(3):445–460. doi: 10.1111/j.1440-1827.1982.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Schliwa M., Porter K. R. Comparison of the three-dimensional organization of unextracted and Triton-extracted human neutrophilic polymorphonuclear leukocytes. Eur J Cell Biol. 1983 Mar;30(1):112–125. [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. Am Rev Respir Dis. 1984 May;129(5):747–753. doi: 10.1164/arrd.1984.129.5.747. [DOI] [PubMed] [Google Scholar]
- Soler P., Basset F., Mazin F., Grandsaigne M., Breton-Gorius J. Peroxidatic activities of human alveolar macrophages in some pulmonary granulomatous disorders. J Reticuloendothel Soc. 1982 Jun;31(6):511–521. [PubMed] [Google Scholar]
- Soranzo M. R., Koerten H. K., Daems W. T. Peroxidatic activity and morphometric analysis of alveolar macrophages in the guinea pig. J Reticuloendothel Soc. 1978 May;23(5):343–359. [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]