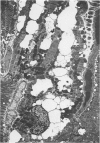
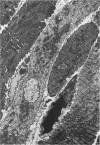
Abstract
Low-grade chronic cardiotoxicity as determined by myocardial biopsy specimens was induced in beagle dogs after four courses of doxorubicin hydrochloride (1.64 mg/kg, 34.0 mg/sq m) given intravenously once every 3 weeks. After this initial treatment, these dogs were separated into three groups. Two groups received six courses of mitoxantrone (0.25 mg/kg, 5 mg/sq m) commencing at 7 weeks or 19 weeks after the final doxorubicin treatment. The third group was treated with six additional courses of doxorubicin after an interval of 7 weeks. Up to seven sequential endomyocardial biopsies were performed to monitor the myocardial changes which were observed after the initial doxorubicin treatment. The low-grade cardiotoxic changes progressed for at least 7-11 weeks without any additional doxorubicin treatment, and stabilized or even partially reversed after 19 weeks of a treatment-free period. Dogs that received additional doxorubicin demonstrated progressive cardiotoxicity, associated with clinical signs, that resulted in death after a total of seven to ten courses of treatment (12-16 mg/kg, 238-340 mg/sq m cumulative dose). In dogs treated with doxorubicin followed by mitoxantrone after a 19-week treatment-free period, myocardial changes were shown to have stabilized and/or partially regressed. This study indicated that in beagle dogs four courses of doxorubicin (7 mg/kg, 136 mg/sq m cumulative dose) are the threshold dose at which non-life-threatening cardiotoxicity occurs. Residual toxic effects of doxorubicin may be erroneously interpreted as adverse findings attributable to other agents given subsequently during the susceptible period, ie, prior to stabilization of the myocardium. Mitoxantrone given after stabilization of doxorubicin-induced low-grade myocardial changes did not show additive or synergistic effects.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingham M. E., Mason J. W., Bristow M. R., Daniels J. R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978 Jun;62(6):865–872. [PubMed] [Google Scholar]
- Henderson B. M., Dougherty W. J., James V. C., Tilley L. P., Noble J. F. Safety assessment of a new anticancer compound, mitoxantrone, in beagle dogs: comparison with doxorubicin. I. Clinical observations. Cancer Treat Rep. 1982 May;66(5):1139–1143. [PubMed] [Google Scholar]
- Murdock K. C., Child R. G., Fabio P. F., Angier R. B., Wallace R. E., Durr F. E., Citarella R. V. Antitumor agents. 1. 1,4-Bis[(aminoalkyl)amino]-9,10-anthracenediones. J Med Chem. 1979 Sep;22(9):1024–1030. doi: 10.1021/jm00195a002. [DOI] [PubMed] [Google Scholar]
- Neidhart J. A., Gochnour D., Roach R. W., Steinberg J. A., Young D. Mitoxantrone versus doxorubicin in advanced breast cancer: a randomized cross-over trial. Cancer Treat Rev. 1983 Dec;10 (Suppl B):41–46. doi: 10.1016/0305-7372(83)90021-x. [DOI] [PubMed] [Google Scholar]
- Paciucci P. A., Cuttner J., Holland J. F. Mitoxantrone as a single agent and in combination chemotherapy in patients with refractory acute leukemia. Semin Oncol. 1984 Sep;11(3 Suppl 1):36–40. [PubMed] [Google Scholar]
- Paciucci P. A., Ohnuma T., Cuttner J., Silver R. T., Holland J. F. Mitoxantrone in acute lymphoblastic leukemia. Cancer Treat Rev. 1983 Dec;10 (Suppl B):65–68. doi: 10.1016/0305-7372(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Sparano B. M., Gordon G., Hall C., Iatropoulos M. J., Noble J. F. Safety assessment of new anticancer compound, mitoxantrone, in beagle dogs: comparison with doxorubicin. II. Histologic and ultrastructural pathology. Cancer Treat Rep. 1982 May;66(5):1145–1158. [PubMed] [Google Scholar]
- Wallace R. E., Murdock K. C., Angier R. B., Durr F. E. Activity of a novel anthracenedione, 1,4-dihydroxy-5,8-bis(((2-[(2-hydroxyethyl)amino]ethyl)amino])-9,10-anthracenedione dihydrochloride, against experimental tumors in mice. Cancer Res. 1979 May;39(5):1570–1574. [PubMed] [Google Scholar]