Abstract
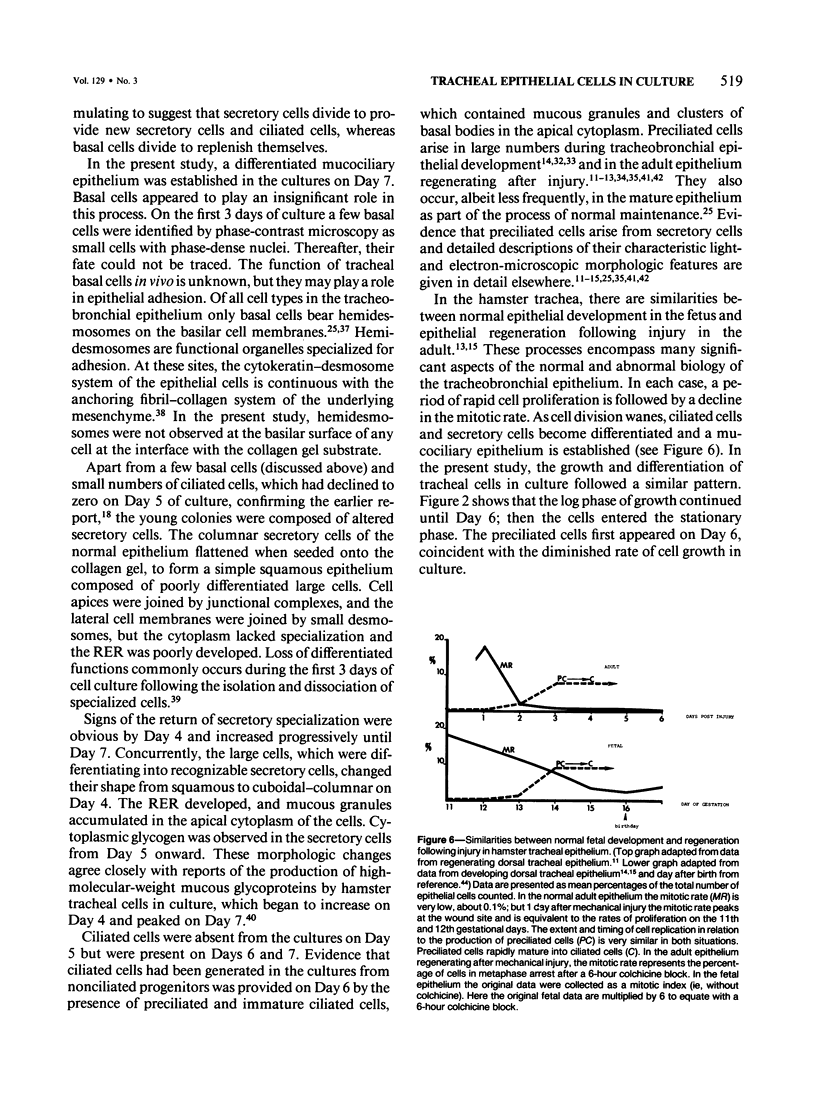
Hamster tracheal epithelial cells were grown in primary culture for 8 days on a collagen gel substrate, in hormone-supplemented serum-free medium (Ham's F-12). On Days 1-3 in culture, the colonies were composed of a monolayer of poorly-differentiated flattened cells. Most of these were large cells which appeared to be altered secretory (mucous) cells. On Days 4 and 5, many of the epithelial cells were cuboidal. The rough endoplasmic reticulum was moderately developed, and mucous granules were seen at the cell apices. Preciliated and newly formed ciliated cells were observed on Day 6, and a differentiated mucociliary epithelium was established by Day 7 in culture. The study shows that in the hamster tracheal epithelium, the stages of normal fetal development and regeneration following injury, which have been characterized previously in vivo, are recapitulated in vitro. Formation of a mucociliary tracheal epithelium occurs within 7 days in vito and in vitro.
Full text
PDF


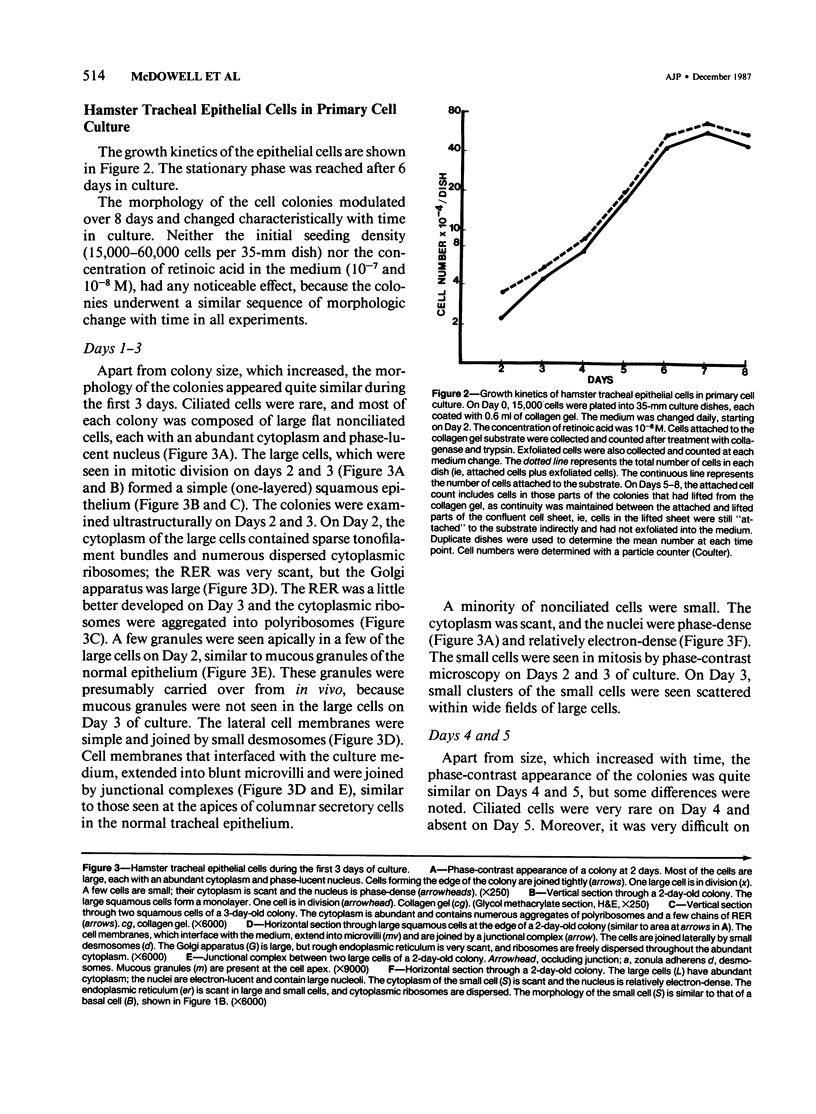





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., Tavassoli M. OTO method for preservation of actin filaments in electron microscopy. J Histochem Cytochem. 1981 May;29(5):682–683. doi: 10.1177/29.5.6894766. [DOI] [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. II. Hamster trachea, bronchus, and bronchioles. J Natl Cancer Inst. 1978 Aug;61(2):551–561. [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. VI. Histogenesis of lung tumors induced by benzo[a]pyrene-ferric oxide in the hamster. J Natl Cancer Inst. 1978 Aug;61(2):607–618. [PubMed] [Google Scholar]
- Chang L. Y., Wu R., Nettesheim P. Morphological changes in rat tracheal cells during the adaptive and early growth phase in primary cell culture. J Cell Sci. 1985 Mar;74:283–301. doi: 10.1242/jcs.74.1.283. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Shami S. G., Cabral-Anderson L. J., Dekker N. P. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986 Apr;123(1):126–133. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S., von Bassewitz D. B., Grundmann E., Nakhosteen J. A., Müller K. M. The ultrastructural heterogeneity of potentially preneoplastic lesions in the human bronchial mucosa. Pathol Res Pract. 1986 Aug;181(4):408–417. doi: 10.1016/S0344-0338(86)80076-0. [DOI] [PubMed] [Google Scholar]
- Gordon R. E., Lane B. P. Ciliated cell differentiation in regenerating rat tracheal epithelium. Lung. 1984;162(4):233–243. doi: 10.1007/BF02715651. [DOI] [PubMed] [Google Scholar]
- Groelke J. W., Coalson J. J., Baseman J. B. Growth requirements of ferret tracheal epithelial cells in primary culture. Proc Soc Exp Biol Med. 1985 Jul;179(3):309–317. doi: 10.3181/00379727-179-42102. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Ayers M., Rogers D. The mechanisms and control of bronchial mucous cell hyperplasia. Adv Exp Med Biol. 1982;144:399–409. doi: 10.1007/978-1-4615-9254-9_62. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Anchoring fibrils in the normal canine respiratory system. Am Rev Respir Dis. 1979 Sep;120(3):595–611. doi: 10.1164/arrd.1979.120.3.595. [DOI] [PubMed] [Google Scholar]
- Keenan K. P., Combs J. W., McDowell E. M. Regeneration of hamster tracheal epithelium after mechanical injury. I. Focal lesions: quantitative morphologic study of cell proliferation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):193–214. doi: 10.1007/BF02890281. [DOI] [PubMed] [Google Scholar]
- Keenan K. P., Combs J. W., McDowell E. M. Regeneration of hamster tracheal epithelium after mechanical injury. II. Multifocal lesions: stathmokinetic and autoradiographic studies of cell proliferation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):215–229. doi: 10.1007/BF02890282. [DOI] [PubMed] [Google Scholar]
- Keenan K. P., Combs J. W., McDowell E. M. Regeneration of hamster tracheal epithelium after mechanical injury. III. Large and small lesions: comparative stathmokinetic and single pulse and continuous thymidine labeling autoradiographic studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):231–252. [PubMed] [Google Scholar]
- Keenan K. P., Wilson T. S., McDowell E. M. Regeneration of hamster tracheal epithelium after mechanical injury. IV. Histochemical, immunocytochemical and ultrastructural studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;43(3):213–240. doi: 10.1007/BF02932958. [DOI] [PubMed] [Google Scholar]
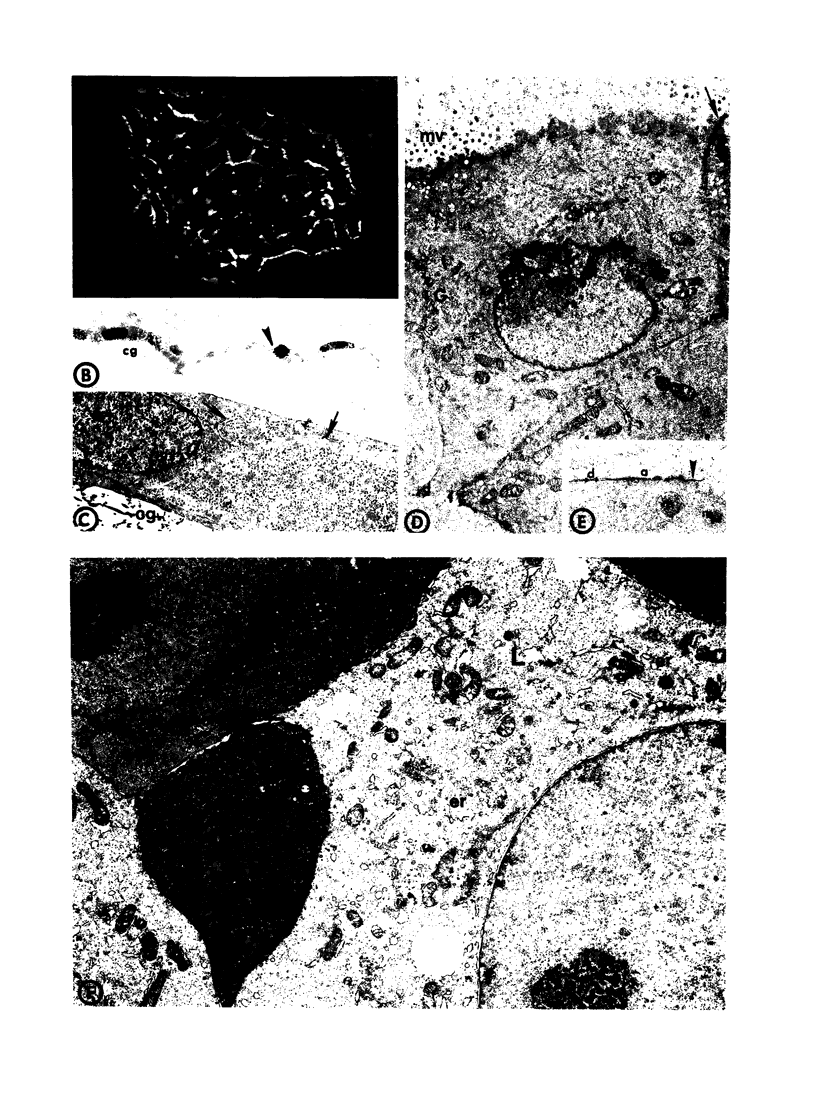
- Kennedy A. R., Desrosiers A., Terzaghi M., Little J. B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J Anat. 1978 Mar;125(Pt 3):527–553. [PMC free article] [PubMed] [Google Scholar]
- Kim K. C., Rearick J. I., Nettesheim P., Jetten A. M. Biochemical characterization of mucous glycoproteins synthesized and secreted by hamster tracheal epithelial cells in primary culture. J Biol Chem. 1985 Apr 10;260(7):4021–4027. [PubMed] [Google Scholar]
- McDowell E. M., Barrett L. A., Glavin F., Harris C. C., Trump B. F. The respiratory epithelium. I. Human bronchus. J Natl Cancer Inst. 1978 Aug;61(2):539–549. [PubMed] [Google Scholar]
- McDowell E. M., Becci P. J., Schürch W., Trump B. F. The respiratory epithelium. VII. Epidermoid metaplasia of hamster tracheal epithelium during regeneration following mechanical injury. J Natl Cancer Inst. 1979 Apr;62(4):995–1008. [PubMed] [Google Scholar]
- McDowell E. M., Combs J. W., Newkirk C. A quantitative light and electron microscopic study of hamster tracheal epithelium with special attention to so-called intermediate cells. Exp Lung Res. 1983 Apr;4(3):205–226. doi: 10.3109/01902148309046061. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Keenan K. P., Huang M. Restoration of mucociliary tracheal epithelium following deprivation of vitamin A. A quantitative morphologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):221–240. doi: 10.1007/BF02889866. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., McLaughlin J. S., Merenyl D. K., Kieffer R. F., Harris C. C., Trump B. F. The respiratory epithelium. V. Histogenesis of lung carcinomas in the human. J Natl Cancer Inst. 1978 Aug;61(2):587–606. [PubMed] [Google Scholar]
- McDowell E. M., Newkirk C., Coleman B. Development of hamster tracheal epithelium: I. A quantitative morphologic study in the fetus. Anat Rec. 1985 Nov;213(3):429–447. doi: 10.1002/ar.1092130309. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Newkirk C., Coleman B. Development of hamster tracheal epithelium: II. Cell proliferation in the fetus. Anat Rec. 1985 Nov;213(3):448–456. doi: 10.1002/ar.1092130310. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
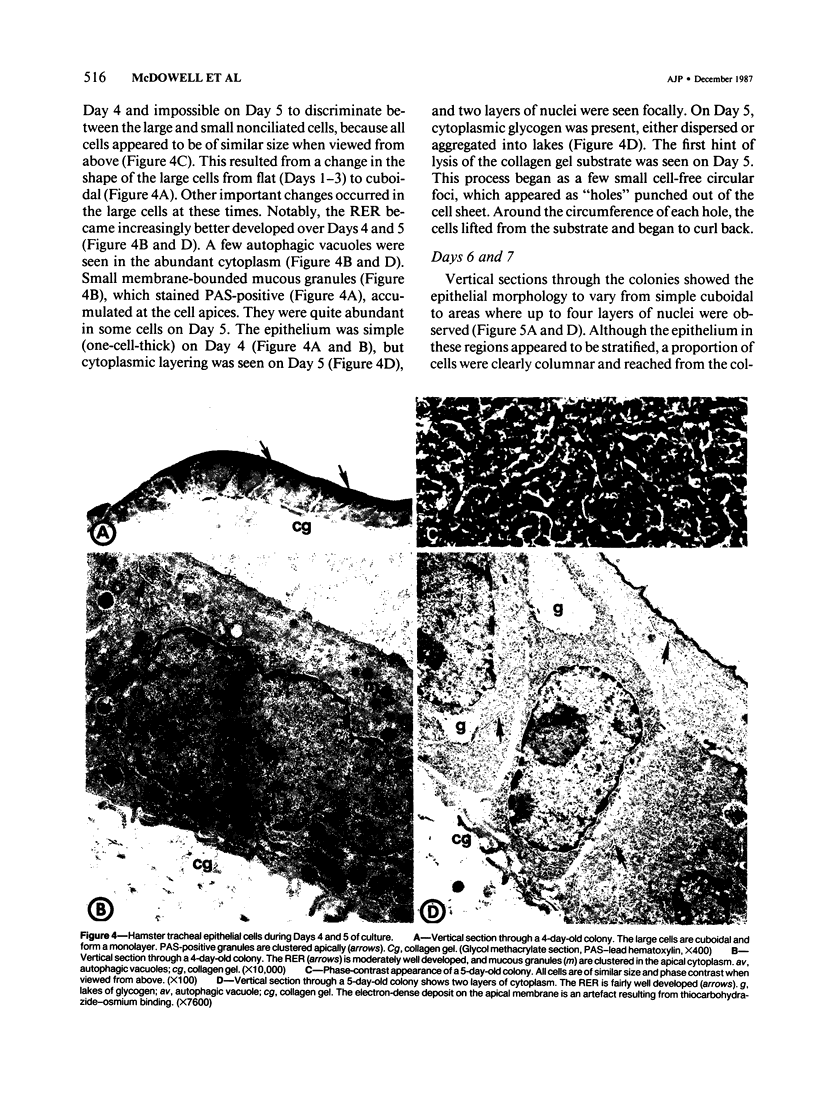
- Otani E. M., Newkirk C., McDowell E. M. Development of hamster tracheal epithelium: IV. Cell proliferation and cytodifferentiation in the neonate. Anat Rec. 1986 Feb;214(2):183–192. doi: 10.1002/ar.1092140213. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Alley J. L., Weir A. J. Differentiation of tracheal epithelium during fetal lung maturation in the rhesus monkey Macaca mulatta. Am J Anat. 1986 Jan;175(1):59–71. doi: 10.1002/aja.1001750107. [DOI] [PubMed] [Google Scholar]
- Ruddell C. L. Embedding media for 1-2 micron sectioning. 2. Hydroxyethyl methacrylate combined with 2-butoxyethanol. Stain Technol. 1967 Sep;42(5):253–255. doi: 10.3109/10520296709115020. [DOI] [PubMed] [Google Scholar]
- Saffiotti U., Cefis F., Kolb L. H. A method for the experimental induction of bronchogenic carcinoma. Cancer Res. 1968 Jan;28(1):104–124. [PubMed] [Google Scholar]
- Sigler R. E., Jones R. T., Hebel J. R., McDowell E. M. Hamster tracheal organ culture in serum-free media: a quantitative comparison of in vitro epithelial morphology with that of in vivo controls. In Vitro Cell Dev Biol. 1987 Feb;23(2):100–110. doi: 10.1007/BF02623589. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Hoyt R. F., Jr PAS-lead hematoxylin as a stain for small-granule endocrine cell populations in the lungs, other pharyngeal derivatives and the gut. Anat Rec. 1978 Oct;192(2):245–259. doi: 10.1002/ar.1091920205. [DOI] [PubMed] [Google Scholar]
- Van Scott M. R., Yankaskas J. R., Boucher R. C. Culture of airway epithelial cells: research techniques. Exp Lung Res. 1986;11(2):75–94. doi: 10.3109/01902148609063272. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Plopper C. G., Dungworth D. L. The response of the macaque tracheobronchial epithelium to acute ozone injury. A quantitative ultrastructural and autoradiographic study. Am J Pathol. 1984 Aug;116(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Tata J. R. Primary culture, cellular stress and differentiated function. FEBS Lett. 1984 Oct 15;176(1):8–15. doi: 10.1016/0014-5793(84)80902-3. [DOI] [PubMed] [Google Scholar]
- Wu R., Nolan E., Turner C. Expression of tracheal differentiated functions in serum-free hormone-supplemented medium. J Cell Physiol. 1985 Nov;125(2):167–181. doi: 10.1002/jcp.1041250202. [DOI] [PubMed] [Google Scholar]