Abstract
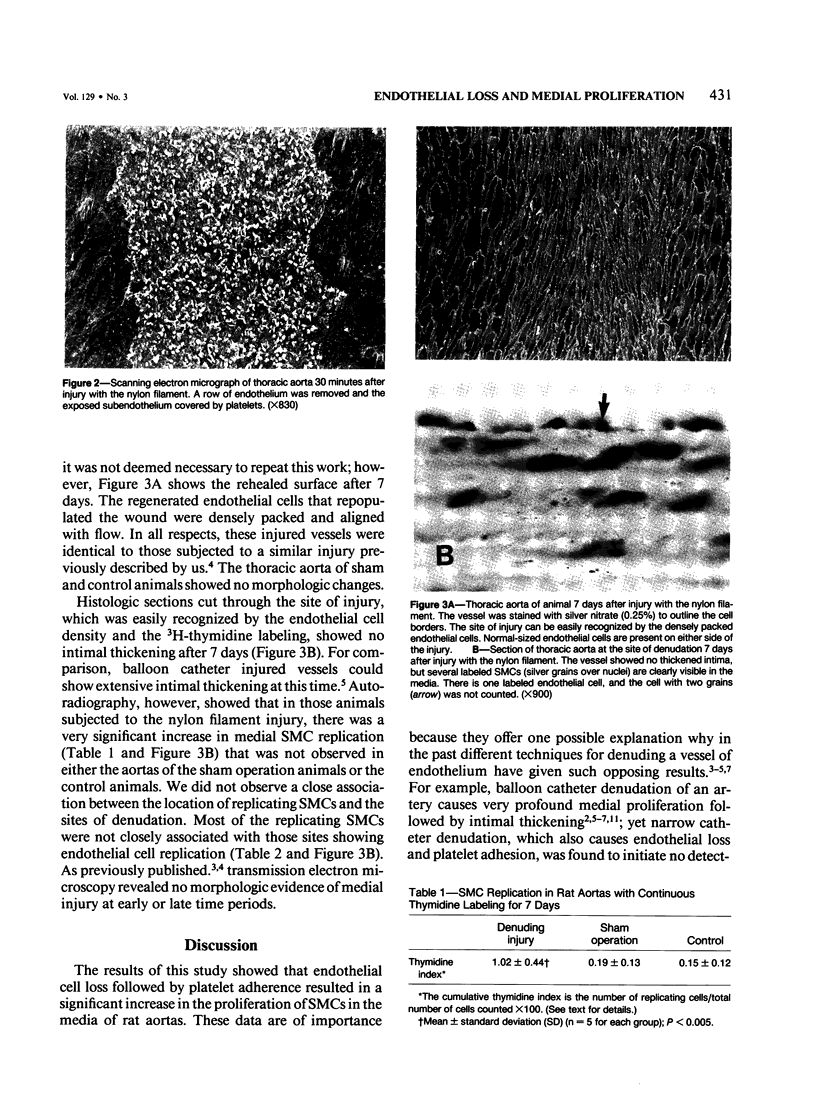
Rat thoracic aortas were denuded of endothelial cells with a fine nylon filament, which removed a row of endothelium approximately 100 mu in width and caused platelets to adhere to the exposed subendothelium. A further group of rats was subjected to a sham operation where only the abdominal aorta was injured, and another group of rats was used as controls. Each animal was continuously labeled with 3H-thymidine (6.7 Ci/mM, 10 microCi/hr) for 7 days, at which time they were killed and the aortas perfusion-fixed with aldehydes. Sections of each aorta were processed for autoradiography. The thoracic aortas from animals subjected to the nylon filament injury showed no intimal thickening, but there was a significant increase in the thymidine index of the medial smooth muscle cells (SMCs) as compared with aortas from sham operation or control animals (1.02% +/- 0.44% versus 0.19% +/- 0.13% versus 0.15% +/- 0.12%). This experiment showed that continuous administration of 3H-thymidine permitted the detection of a small but significant increase in replication rates that could not be detected by the standard bolus administration of 3H-thymidine, and that selected loss of endothelium with minimal trauma to the vessel wall caused SMC proliferation without intimal thickening. These findings suggest that platelets may indeed provide SMC proliferation, but that migration of these cells into the intima may be controlled by different factor(s).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Favreau L. V., Karnovsky M. J., Rosenberg R. D. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982 Oct 10;257(19):11256–11260. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Reidy M. A., Silver M. Endothelial regeneration. VII. Lack of intimal proliferation after defined injury to rat aorta. Am J Pathol. 1985 Feb;118(2):173–177. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Studies on aortic intima. I. Structure and permeability of rat thoracic aortic intima. Am J Pathol. 1972 Feb;66(2):241–264. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B., Veith F. J., Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975 Jan;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]




