Abstract
We carried out a cross-sectional study to investigate antimicrobial resistance patterns of Campylobacter coli isolated from Ontario grower-finisher pigs. From January to June 2004, 1200 samples were collected from 80 farms by obtaining a constant number (15) of fecal samples per farm. Susceptibility of the isolates to 11 antimicrobial drugs was determined by the agar-dilution technique. The overall prevalence of resistance to 1 or more antimicrobials among the isolates was 99.2%. High levels of resistance were observed for azithromycin, clindamycin, erythromycin, streptomycin, and tetracycline: 91.7%, 82.5%, 81.4%, 70.7%, and 63.7%, respectively. For sulfamethoxazole, ampicillin, and nalidixic acid, resistance was observed in 40.3%, 26.6%, and 22.7% of the isolates, respectively. Although at very low levels, resistance was observed for ciprofloxacin (a fluoroquinolone), chloramphenicol, and gentamicin: in 2.4%, 1.7%, and 0.2%, respectively. Many of the isolates (29.7%) were resistant to 5 antimicrobials, the most common being azithromycin, clindamycin, erythromycin, streptomycin, and tetracycline. Isolates from the same farm showed at least 5 patterns of resistance. Results from this study indicate high levels of resistance to the antimicrobial drugs most commonly used in the Canadian swine industry (macrolides, lincosamides, and tetracyclines) among C. coli isolated from grower-finisher pigs in Ontario. Macrolides and fluoroquinolones are the drugs most commonly used to treat severe human campylobacteriosis. Fortunately, at present, there is little resistance to fluoroquinolones among C. coli from pigs in Ontario.
Résumé
Cette étude transversale a permis d’étudier les patrons de résistance aux antimicrobiens d’isolats de Campylobacter coli provenant de porcs de l’Ontario en période de croissance-finition. De janvier à juin 2004, 1200 échantillons ont été prélevés sur 80 fermes en sélectionnant un nombre constant (15) d’échantillons de fèces sur chaque ferme. La sensibilité des isolats à 11 agents antimicrobiens a été déterminée par la technique de dilution en gélose. La prévalence globale de la résistance à 1 antimicrobien ou plus parmi les isolats était de 99,2 %. De hauts degrés de résistance ont été observés pour l’azithromycine, la clindamycine, l’érythromycine, la streptomycine, et la tétracycline, soit respectivement 91,7 %, 82,5 %, 81,4 %, 70,7 %, et 63,7 %. Pour le sulfaméthoxazole, l’ampicilline, et l’acide nalidixique, les degrés de résistance observés étaient respectivement de 40,3 %, 26,6 %, et 22,7 %. Bien qu’à des degrés peu élevés, soit respectivement 2,4 %, 1,7 %, et 0,2 %, de la résistance a été observée pour le ciprofloxacin (une fluoroquinolone), le chloramphénicol, et la gentamicine. Plusieurs isolats (29,7 %) étaient résistants à 5 antimicrobiens, les plus communs étant l’azithromycine, la clindamycine, l’érythromycine, la streptomycine, et la tétracycline. Des isolats provenant de la même ferme ont montré au moins 5 patrons de résistance. Les résultats de cette étude indiquent de hauts degrés de résistance aux agents antimicrobiens les plus couramment utilisés dans l’industrie porcine canadienne (macrolides, lincosamides, et tétracyclines) chez des isolats de C. coli provenant de porcs de l’Ontario en période de croissance-finition. Les macrolides et les fluoroquinolones sont les antimicrobiens utilisés de routine pour traiter les campylobactérioses humaines sévères. Heureusement, il n’y a pas pour l’instant de résistance aux fluoroquinolones parmi les C. coli provenant de porcs de l’Ontario.
(Traduit par Docteur Serge Messier)
Introduction
Increasing resistance to antimicrobial agents is an important public health concern worldwide. Foodborne infections with resistant pathogens have emerged as a threat to human health. Campylobacter spp. are the leading cause of foodborne bacterial gastroenteritis in the United States, affecting nearly 2.4 million people annually (1). Although most Campylobacter infections are self-limiting, complications may occur and antimicrobial therapy may be required. Fluoroquinolones and macrolides are the drugs of choice for people with severe campylobacteriosis. These antimicrobials may also be used to eliminate the carrier state of Campylobacter infection (2). Antimicrobial resistance in Campylobacter isolated from humans is suspected of being connected to antimicrobial drug use in food-producing animals (3,4), and, therefore, several studies have been conducted to discover the extent of the resistance in isolates recovered from farms. Some of the antimicrobials used in human and animal medicine are last-line drugs for treatment of serious human infections, and alternative antimicrobials are not available to treat human infections caused by resistant bacteria. Antimicrobials have been classified according to their importance to human medicine as follows: category I, antimicrobials of very high importance (VHI), used for the treatment of life-threatening bacterial infections; category II, antimicrobials of high importance (HI), used to treat infections caused by bacteria resistant to category III agents; category III, antimicrobials of medium importance (MI), used as 1st-line drugs for the treatment of bacterial infections; and category IV, antimicrobials of low importance, of limited use in human medicine (5). Emerging resistance to macrolides, tetracyclines, and fluoroquinolones has been observed in numerous countries (1,6,7).
The poultry industry has often been considered to be primarily responsible for human campylobacteriosis; however, the pork industry has also been identified as a potential source of human infection (4,8). In 1988, the emergence of fluoroquinolone resistance in Campylobacter strains was first reported in Spain, and since then the emergence of fluoroquinolone resistance has been identified in many countries, including Finland, the Netherlands, England, and Canada (9). In Spain, 100% of Campylobacter strains isolated from broilers and pigs were found to be resistant to fluoroquinolones (9). The main objective of the study reported here was to establish the pattern of antimicrobial resistance in Campylobacter isolated from swine in Ontario. The 2nd objective was to determine the variation in antimicrobial resistance between and within farms.
Materials and methods
Farm selection and visit
Eighty farms were selected, some from operations conveniently close to Guelph, Ontario, others on the basis of the geographic distribution of farms in Ontario and herd type, and still others from operations of swine producers willing to participate after the end of a previous study. The process of selection was not a true random sampling; however, the swine operations represented farms from all the swine-producing regions of southern Ontario and in terms of management style ranged from single-site farrow-to-finish operations to specialized large, multisite operations with direct following of pigs. The farms were visited in 2004 between January and June, and 15 samples were collected from each farm by a standard procedure, for a total of 1200 samples.
Fecal sampling and processing
For each all-in/all-out grower-finisher barn, 5 pens were randomly selected and 15 specimens collected. In herds managed with a continuous-flow system in the grower-finisher barn, the 15 specimens were collected from the 5 pens identified as having the largest pigs. In each pen, 2 samples obtained per rectum from 2 hogs were collected; a 3rd sample was obtained by combining fecal samples from 5 different places in the pen (pooled environmental sample). Samples were stored in sterile containers (Starplex Scientific, Etobicoke, Ontario), transported to the Laboratory Services Division, University of Guelph, in coolers containing ice packs, and processed within 24 h of collection.
Campylobacter isolation and categorization
The following procedures were used to isolate and categorize Campylobacter.
Enrichment in a selective broth
A 10-g aliquot of each fecal sample was mixed with 90 mL of 0.1% buffered peptone water (Oxoid, Ottawa, Ontario) and homogenized in a sterile stomacher bag. Next, 1 mL of the rinse was added to each 9-mL tube of Hunt Enrichment Broth (HEB), and 2 subsequent dilutions were prepared in HEB tubes. All the tubes, including controls, were placed in microaerophilic conditions (5% O2, 10% CO2, and 85% N2) and incubated at 37°C ± 1.0°C while being shaken at 25 × g for 4 h. After incubation, 36 μL of sterile cefoperazone solution was added to bring the final concentration to 30 mg/L. The microaerobic atmosphere was then re-established and the solution incubated at 42°C ± 1.0°C for 24 h while being shaken at 25 × g.
Selective plating
Modified Campylobacter Charcoal Differential Agar (MCCDA) plates containing cefoperazone and amphotericin B were inoculated with serial enrichments and incubated at 42°C in microaerophilic conditions for 48 h. Well-isolated typical colonies were selected from each plate and examined by dark-field microscopy. Campylobacter colonies on MCCDA are either smooth, shiny, and convex, with a defined edge, or flat, translucent, and spread-out, with an irregular edge. They are usually colorless or else greyish or light cream, and are usually 1 to 2 mm in diameter but may grow to several millimetres in diameter. The following medium controls were inoculated with each batch of tests to ensure proper medium formulation and sterility, as well as atmospheric conditions: positive control, C. jejuni American Type Culture Collection (ATCC) 49432; negative control, Escherichia coli ATCC 25922; and blank control, no bacteria.
Biochemical characterization
A biotyping scheme was used to differentiate between the 3 classic thermophilic Campylobacter spp: C. jejuni, C. coli, and C. lari. Hippurate hydrolysis, production of hydrogen sulfide, DNA hydrolysis, and indoxyl acetate hydrolysis were evaluated in colonies suspected to be Campylobacter. Colonies able to hydrolyze both hippurate and indoxyl acetate were classified as C. jejuni, those able to hydrolyze indoxyl acetate but not hippurate were classified as C. coli, and those able to hydrolyze neither hippurate nor indoxyl acetate were classified as C. lari. This procedure was based on the method used by Health Canada’s National Laboratory for Enteric Pathogens, which was described in 1984 by Lior (10) and later revised by Lior and Patel (11). Colonies identified as C. coli were inoculated into 1.5-mL microtubes (Sarstedt, Montreal, Quebec) with 0.6 mL of Mueller-Hinton broth (Difco Laboratories, Detroit, Michigan, USA) containing 50% glycerol (Fisher Scientific, Ottawa, Ontario) and stored at −70°C for future susceptibility testing.
Antimicrobial susceptibility testing
Only isolates identified as C. coli were tested for antimicrobial susceptibility. Susceptibility was tested for a range of antimicrobials (VHI, HI, or MI) commonly used in human therapy, at the range of concentrations approved for susceptibility testing of Campylobacter spp. by the US Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee for Clinical Laboratory Standards) (12), listed by the US Food and Drug Administration (FDA), and used by the US National Antimicrobial Resistance Monitoring System (NARMS) and the US Centers for Disease Control (13). The breakpoint minimum inhibitory concentrations (MICs), used to categorize the isolates as resistant to a given antimicrobial and also based on the CLSI standard, were as follows: azithromycin, ≥ 2 μg/mL; ciprofloxacin and clindamycin, ≥ 4 μg/mL; erythromycin, ≥ 8 μg/mL; gentamicin and tetracycline, ≥ 16 μg/mL; nalidixic acid, ampicillin, and chloramphenicol, ≥ 32 μg/mL; streptomycin, ≥ 64 μg/mL; and sulfamethoxazole, ≥ 512 μg/mL.
The isolates were tested by the agar-dilution method with use of the Cathra Replianalyzer system (Oxoid) according to the manufacturer’s instructions. After biochemical identification, the frozen colony suspensions were streaked onto the surface of Mueller-Hinton blood agar (MHBA) plates supplemented with 5% defibrinated sheep blood and incubated at 37°C for 48 h in a microaerophilic environment for 2 passages. Growth from the plates was suspended in Muller-Hinton broth at 4°C to give turbidity equal to that of a 0.5 McFarland standard. The suspensions were dispensed into the wells of a cold Cathra replicator 36-well plate in the following sequence: the 1st well was filled with 300 μL of India ink, the next 3 wells were each filled with 300 μL of 3 reference strains (E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and C. jejuni ATCC 33560), and the last 33 wells were filled with suspensions of C. coli. Suspensions were spotted onto MHBA plates containing the antimicrobial agents tested. Each inoculated plate was dried at room temperature and microaerophilically incubated at 42°C for 48 h. The MIC was taken as the lowest concentration of the antimicrobial at which there was no visible growth.
Data handling and statistical analysis
Data were entered into an Excel 2000 spreadsheet (Microsoft, Redmond, Washington, USA) and subsequently imported into SAS 8, version 8 (SAS Institute, Cary, North Carolina, USA). For statistical analysis all the isolates were classified as susceptible, of intermediate susceptibility, or resistant. Descriptive statistics were performed to generate tables and graphs illustrating the general trends in resistance.
Results
Of the 1200 samples, Campylobacter was isolated from 1194, of which 1185 yielded C. coli. A small number of isolates would not grow on subsequent inoculation and, therefore, 1149 isolates were tested for susceptibility. The overall prevalence of resistance to 1 or more antimicrobials was 99.2% (1140 of 1149), corresponding to 380 of 385 environmental isolates and 760 of 764 pig isolates. None of the isolates were resistant to all 11 antimicrobials; 9 isolates (0.8%), of which 5 (1.3%) were environmental and 4 (0.5%) pig isolates, were sensitive to all 11 antimicrobials. Resistance to 9 antimicrobials (ampicillin, azithromycin, ciprofloxacin, clindamycin, erythromycin, nalidixic acid, streptomycin, sulfamethoxazole, and tetracycline) was observed in only 4 isolates (0.3%).
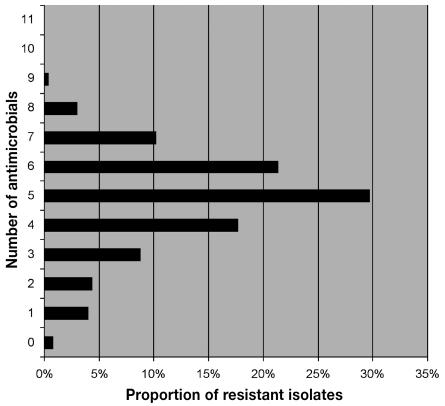
About one-third of the isolates (29.7%; 341 of 1149) were resistant to 5 antimicrobials, most commonly azithromycin, clindamycin, erythromycin, streptomycin, and tetracycline. Different patterns of resistance were observed on each farm; isolates from 1 farm showed at least 5 patterns. In addition, the patterns in samples from individual pigs were dissimilar from the pattern obtained from environmental samples (data not shown).
The overall prevalence of resistance, as well as the prevalence at different levels (pig, environmental, and farm), are presented in Table I and Figure 1. A high proportion of isolates were resistant to azithromycin, clindamycin, and erythromycin, and somewhat lower proportions to streptomycin and tetracycline. A moderate proportion of isolates were resistant to sulfamethoxazole, ampicillin, and nalidixic acid. The proportion of isolates resistant to ciprofloxacin (2.4%), chloramphenicol (1.7%), and gentamicin (0.2%) was very low.
Table I.
Prevalence of resistance to 11 antimicrobials among Campylobacter coli isolates from pigs on 80 grower-finisher farms in Ontario
| Proportion of isolates resistant (%)
|
||||
|---|---|---|---|---|
| Antimicrobial and importance to human health | Overalla (n = 1149) | Herd level (n = 80) | Pen levelb (n = 385) | Pig levelc (n = 764) |
| Very high | ||||
| Ciprofloxacin | 2.4 | 20 | 2.3 | 2.4 |
| High | ||||
| Azithromycin | 91.7 | 100 | 92.0 | 91.6 |
| Clindamycin | 82.5 | 99 | 82.6 | 82.5 |
| Erythromycin | 81.4 | 99 | 81.0 | 81.5 |
| Nalidixic acid | 22.7 | 79 | 23.6 | 22.2 |
| Moderate | ||||
| Ampicillin | 26.6 | 85 | 27.5 | 26.2 |
| Chloramphenicol | 1.7 | 13 | 1.6 | 1.8 |
| Gentamicin | 0.2 | 1 | 0.3 | 0.1 |
| Streptomycin | 70.7 | 100 | 75.1 | 68.5 |
| Sulfamethoxazole | 40.3 | 89 | 38.7 | 41.1 |
| Tetracycline | 63.7 | 91 | 64.4 | 63.4 |
For pen- and pig-level samples combined.
For each pen, a pooled sample of feces collected from 5 locations was analyzed.
For each pig, isolates from a freshly voided sample collected per rectum were analyzed.
Figure 1.
Prevalence of resistance to multiple antimicrobials among Campylobacter coli isolated from pigs in Ontario.
The level of resistance was high for macrolides (azithromycin, 91.7%; erythromycin, 81.4%) but not for fluoroquinolones (ciprofloxacin, 2.4%). Among quinolones, the prevalence of resistance was higher for nalidixic acid (22.7%) than for ciprofloxacin (2.4%); 63 farms had 1 or more isolates resistant to nalidixic acid, whereas 16 farms had 1 or more isolates resistant to ciprofloxacin, with 13 of the 16 having only 1 resistant isolate per farm, and the other 3 having a total of 11 resistant isolates. Of the 27 isolates resistant to ciprofloxacin, 24, from 14 farms, were also resistant to nalidixic acid.
Discussion
Although Campylobacter infections in humans are typically self-limiting, severe cases may occur, in which antimicrobial therapy is required (7). During the last decade the occurrence of strains of zoonotic pathogens that cause disease in humans and are resistant to multiple drugs has increased and is limiting effective treatment of human infections (14). This study was designed to assess the patterns of antimicrobial resistance of C. coli isolates from grower-finisher pig farms in Ontario. Fluoroquinolones (ciprofloxacin) and macrolides (erythromycin and azithromycin), classified as category I and category II antimicrobials, respectively, are of particular importance since patients with severe campylobacteriosis are generally treated with these agents; ampicillin and other β-lactam antibiotics are not recommended because of a high incidence of resistance to this family of drugs (15). In this study, 26.6% of the isolates were resistant to ampicillin, a rate comparable to those reported from France and Denmark (20% and 17%, respectively) but lower than that reported from Spain (65.7%). Resistance to β-lactam antimicrobials in pathogenic bacteria develops through bacterial conjugation. This resistance-transfer mechanism is important because it permits genetic exchange of information between species of bacteria (16).
In this study, we detected resistance frequently to the antimicrobials most commonly used in the Canadian swine industry, reportedly tylosin, lincomycin, tetracycline, and sulfamethoxazole in Ontario (17,18). Different susceptibility patterns were observed for the 2 aminoglycoside antimicrobials: of all the isolates we tested, only 2 (0.2%), from 1 farm, were resistant to gentamicin, but 70.7% were resistant to streptomycin. These results are comparable to those reported from Quebec, Denmark, and Switzerland, where no resistance to gentamicin was observed, whereas resistance to streptomycin was frequent (4,6,19). Gentamicin has been recommended as an alternative for the treatment of human campylobacteriosis (20). Resistance to drugs like streptomycin that have not been licensed for use in food animals in Ontario for years could be attributed to cross-resistance due to the common use of other aminoglycosides, such as neomycin, apramycin, and spectinomycin, to treat nursery or grower-finisher pigs.
Higher levels of macrolide resistance have been reported among C. coli isolates than among C. jejuni isolates. Saenz et al (9) reported no erythromycin resistance among C. jejuni isolates from broilers and high levels of resistance among C. coli isolates from pigs (81.1%) compared with those from humans (34.5%). With surveillance data from 1997 to 2001, NARMS showed that 95% of Campylobacter spp. isolates from humans were C. jejuni and 4% C. coli; 13% of the C. coli isolates but only 0.14% of the C. jejuni isolates were resistant to erythromycin. A recent study in Quebec found that 61% of C. coli isolates from pigs but no C. coli isolates from chickens were resistant to erythromycin (4). Our results confirm the high prevalence of macrolide resistance among C. coli isolates: 81.4% for erythromycin and 91.7% for azithromycin in this study. A similar pattern was reported for azithromycin in the Quebec study.
High levels of resistance to erythromycin and azithromycin, antimicrobials classified as category II, is of great concern, since treatment of human Campylobacter infections may be compromised. Antimicrobials classified as category I are the alternative in cases of resistance to category II antimicrobials. However, some of these agents have unique mechanisms of action and, hence, should be reserved for the treatment of serious infections, since there may be no alternative antimicrobials if resistance to these agents emerges (5).
One explanation for a high rate of macrolide resistance in Ontario C. coli is the widespread use of tylosin in swine rations and the assumption that resistance to tylosin leads to resistance to other macrolides, such as azithromycin and erythromycin (18). Macrolide resistance in C. coli from pigs in Denmark has decreased along with the decreasing use of tylosin since the Danish ban of growth promotants in feed (7).
Resistance to tetracycline, an antimicrobial that has been commonly used in the swine industry for more than 50 y, was more prevalent in our study than was reported by NARMS in 2003 but lower than the rates reported for Spain and France (9,21). In contrast, Denmark and Switzerland reported very low rates of resistance to tetracycline (6,19). The observed levels of sulfamethoxazole resistance may be also explained by the fact that compounds containing sulfonamides have been heavily used in the swine industry for more than 30 y for growth promotion and for therapeutic purposes (17).
Very little resistance was found to fluoroquinolones, represented in our study by ciprofloxacin. This is in contrast to European countries, where isolates of C. coli from pigs are often resistant to fluoroquinolones. A recent Spanish study suggests an extremely high prevalence of ciprofloxacin resistance in Campylobacter strains isolated from broilers (99%), pigs (100%), and human feces (72%) (9). Our results imply that resistance to fluoroquinolones, an antimicrobial group of highest importance in human medicine, is still very low for C. coli. Cross-resistance between nalidixic acid and ciprofloxacin was found in all the quinolone-resistant strains tested in Spain (9). Nevertheless, the rates of resistance to nalidixic acid and ciprofloxacin among our tested isolates were 22.7% and 2.4%, respectively, showing that although there was moderately frequent resistance to nalidixic acid, resistance to fluoroquinolones was still rare, perhaps because fluoroquinolones are not licensed for use in swine in Canada. In various European countries, the use of fluoroquinolones in food animals has been directly linked to the emergence and persistence of ciprofloxacin resistance in Campylobacter (22,23).
The wide use of lincomycin, a lincosamide that is similar to clindamycin, in the Ontario swine industry (18) is likely the reason for the high level of resistance to clindamycin found in our study. In addition, cross-resistance between macrolides and lincosamides can occur by a mechanism known as overlapping targets, which, by addition of a methyl group to a single adenine residue in ribosomal RNA confers high-level resistance to 3 groups of antimicrobials that are distantly chemically related: macrolides, lincosamides, and streptogramins (24). Clindamycin is a therapeutic alternative for campylobacteriosis. In this study, the rate of resistance to clindamycin was higher than the rates reported in the United States (25) and comparable to those reported in Spain (9).
Interestingly, 1.7% of the C. coli isolates in our study exhibited resistance to chloramphenicol, an antimicrobial that has been banned in the swine industry for about 20 y. It has been reported that under certain conditions resistance against some antimicrobials may persist even in the absence of use of the antimicrobial, most likely as a consequence of coselection of resistance genes by the use of different antimicrobials (26).
All herds in this study had some isolates resistant to azithromycin and streptomycin, but isolates from only 1 farm were resistant to gentamicin. In general, we can conclude from these findings that the prevalence of resistance of C. coli isolates at the herd level in Ontario was very high for azithromycin, streptomycin, erythromycin, clindamycin, tetracycline, sulfamethoxazole, ampicillin, and nalidixic acid. Similarly, high resistance to streptomycin, erythromycin, clindamycin, and tetracycline was found in Quebec (4).
Our data illustrate the variability in the antimicrobial resistance pattern on farms in Ontario. Further studies are needed to clarify whether individual animals have exclusive resistance patterns and whether patterns for different bacteria from the same sample are similar. To address this question, it is necessary to test multiple samples per pig and more isolates from a single sample. With a better understanding of the distribution of antimicrobial resistance patterns in Campylobacter spp. isolated from pigs, effective strategies concerning antimicrobial drug use on pig farms can be developed to protect public health.
Our findings support the government action to not license the use of fluoroquinolones in pigs and other food-producing animals in Canada, since such use causes the development of fluoroquinolone resistance in Campylobacter that can be transferred to humans.
Acknowledgments
We acknowledge the financial support of Ontario Pork, the University of Guelph, and the Ontario Ministry of Agriculture, Food and Rural Affairs. We thank the Laboratory Services Division, University of Guelph, for culturing samples and the Ontario pork producers for their cooperation and willingness to participate in this study.
References
- 1.Gupta A, Nelson JM, Barrett TJ, et al. Antimicrobial resistance among Campylobacter strains, United States, 1997–2001. Emerg Infect Dis. 2004;10:1102–1009. doi: 10.3201/eid1006.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Campylobacter WHO fact sheet 255. Geneva: WHO, 2000.
- 3.Vaarst M. Protecting the food chain: food safety, animal and human health and the use of homeopathy in farm animals (www.safonetwork.org/publications/other/index.html). In: Proceedings of the 60th Congress of the Liga Medicorum Homeopathica Internationalis; Berlin, Germany; 2005 May 4–7. Slagelse, Denmark: Sustaining Animal Health and Food Safety in Organic Farming, 2003.
- 4.Guévremont E, Nadeau E, Sirois M, Quessy S. Antimicrobial susceptibilities of thermophilic Campylobacter from humans, swine, and chicken broilers. Can J Vet Res. 2006;70:81–86. [PMC free article] [PubMed] [Google Scholar]
- 5.Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS). Ottawa: Health Canada, 2002. www.hc-sc.gc.ca/pphb-dgspsp/cipars-picra/index.html (accessed 2004 January 23).
- 6.Aarestrup FM, Nielsen EM, Madsen M, Engberg J. Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. from humans, pigs, cattle, and broilers in Denmark. Antimicrob Agents Chemother. 1997;41:2244–2250. doi: 10.1128/aac.41.10.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis. 2001;7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manser PA, Dalziel RW. A survey of Campylobacter in animals. J Hyg (Lond) 1985;95:15–21. doi: 10.1017/s0022172400062239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenz Y, Zarazaga M, Lantero M, Gastanares MJ, Baquero F, Torres C. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997–1998. Antimicrob Agents Chemother. 2000;44:267–71. doi: 10.1128/aac.44.2.267-271.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lior H. New, extended biotyping scheme for Campylobacter jejuni, Campylobacter coli, and “Campylobacter laridis”. J Clin Microbiol. 1984;20:636–640. doi: 10.1128/jcm.20.4.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lior H, Patel A. Improved toluidine blue-DNA agar for detection of DNA hydrolysis by campylobacters. J Clin Microbiol. 1987;25:2030–2031. doi: 10.1128/jcm.25.10.2030-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 2nd ed. Approved Standard M31-A2. Wayne, Pennsylvania: NCCLS, 2002.
- 13.US Department of Agriculture, Agricultural Research Service. National Antimicrobial Resistance Monitoring System — Enteric Bacteria (NARMS–EB). Veterinary Isolates. Final Report. Atlanta, Georgia: FDA/USDA/CDC, 1998.
- 14.Ewen CD, Todd CN. Agriculture, Food Safety, and Foodborne Diseases. Understanding the Links Between Agriculture and Health. Focus 13. Brief 5 of 16. Washington, DC: International Food Policy Research Institute, 2006.
- 15.Navarro F, Miro E, Mirelis B, Prats G. Campylobacter spp antibiotic susceptibility. J Antimicrob Chemother. 1993;32:906–907. doi: 10.1093/jac/32.6.906. [DOI] [PubMed] [Google Scholar]
- 16.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 17.Veterinary Drugs Directorate, Health Canada. Uses of antimicrobial drugs in food animals. In: Uses of Antimicrobials in Food Animals in Canada: Impact on Resistance and Human Health. Report of the Advisory Committee on Animal Uses of Antimicrobials and Impact on Resistance and Human Health. Ottawa, Ontario: Health Canada, 2002.
- 18.Dunlop RH, McEwen SA, Meek AH, Friendship RA, Clarke RC, Black WD. Antimicrobial drug use and related management practices among Ontario swine producers. Can Vet J. 1998;39:87–96. [PMC free article] [PubMed] [Google Scholar]
- 19.Schuppers ME, Stephan R, Ledergerber U, et al. Clinical herd health, farm management and antimicrobial resistance in Campylobacter coli on finishing pig farms in Switzerland. Prev Vet Med. 2005;69:189–202. doi: 10.1016/j.prevetmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez H, Mansilla M, Gonzalez V. Antimicrobial susceptibility of Campylobacter jejuni subsp. jejuni assessed by E-test and double dilution agar method in southern Chile. Mem Inst Oswaldo Cruz. 2000;95:247–249. doi: 10.1590/s0074-02762000000200020. [DOI] [PubMed] [Google Scholar]
- 21.Payot S, Dridi S, Laroche M, Federighi M, Magras C. Prevalence and antimicrobial resistance of Campylobacter coli isolated from fattening pigs in France. Vet Microbiol. 2004;101:91–99. doi: 10.1016/j.vetmic.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Delsol AA, Sunderland J, Woodward MJ, Pumbwe L, Piddock LJ, Roe JM. Emergence of fluoroquinolone resistance in the native Campylobacter coli population of pigs exposed to enrofloxacin. J Antimicrob Chemother. 2004;53:872–874. doi: 10.1093/jac/dkh150. [DOI] [PubMed] [Google Scholar]
- 23.Piddock LJ. Quinolone resistance and Campylobacter spp. J Antimicrob Chemother. 1995;36:891–898. doi: 10.1093/jac/36.6.891. [DOI] [PubMed] [Google Scholar]
- 24.Courvalin P. The antibiotic food-chain gang. Emerg Infect Dis. 2001;7:489–490. doi: 10.3201/eid0703.010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Antimicrobial Resistance Monitoring System: Enteric Bacteria. Bacterial Epidemiology and Antimicrobial Resistance (BEAR). Antimicrobial Resistance of Campylobacter from 1998–2003. Atlanta, Georgia: Centers for Disease Control and Prevention, 2003.
- 26.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]