Abstract
To determine whether Mycoplasma hyopneumoniae colonization at weaning in off-site weaning systems is associated with the severity of respiratory disease due to this agent in growing pigs, we studied 20 groups, each group representing a different week in production, in sow herds at 3 farms of 3000 sows each that had a prevalence of M. hyopneumoniae colonization at weaning higher than 5%. The calculated sample size for assessment at weaning was 39 piglets for each group under study; 39 litters were randomly selected, and 1 piglet was randomly selected from each litter for testing and ear-tagged. In total, 780 piglets were tested. The presence of M. hyopneumoniae in nasal swabs at weaning was established by nested polymerase chain reaction (PCR). All groups were followed until slaughter, at which time blood samples were collected from each ear-tagged pig to test for M. hyopneumoniae antibodies, bronchial swabs were collected for detection of M. hyopneumoniae DNA by nested PCR, and the lung lesion score and percentage of affected lungs in the same animals were calculated. Correlation analyses showed a positive correlation between colonization at weaning and all 4 dependent variables indicating infection at slaughter: average lung lesion score, percentage of affected lungs, presence of M. hyopneumoniae on the bronchial epithelium, and seroconversion. This study provides evidence that severity of the disease can be predicted by the prevalence at weaning in segregated systems. Therefore, strategies focused on reducing colonization at weaning seem to be important elements in the global control of M. hyopneumoniae in segregated production systems.
Résumé
Une étude a été menée afin de déterminer si la colonisation par Mycoplasma hyopneumoniae au moment du sevrage dans les systèmes de production avec sevrage hors-site est associée avec la sévérité des problèmes respiratoires dus à cet agent chez les porcs en croissance. Pour se faire on utilisa 20 groupes, représentant chacun une semaine différente de production, et provenant de troupeaux de truies sur 3 fermes de 3000 truies chacune qui avaient une moyenne de prévalence de colonisation par M. hyopneumoniae au sevrage supérieure à 5 %. La taille calculée de l’échantillonnage pour l’évaluation au sevrage était de 39 porcelets pour chaque groupe à l’étude; 39 portées ont été sélectionnées au hasard, et 1 porcelet de chaque portée identifié a été choisi de manière aléatoire. Au total, 780 porcelets ont été testés. La présence de M. hyopneumoniae à partir d’écouvillons nasaux au sevrage a été établie par réaction d’amplification en chaîne (PCR) nichée. Tous les groupes ont été suivis jusqu’au moment de l’abattage. À ce moment, on a prélevé de chaque porc identifié des échantillons de sang afin de vérifier la présence d’anticorps dirigés contre M. hyopneumoniae, des écouvillons des bronches pour la détection d’ADN de M. hyopneumoniae par PCR nichée, et le pointage des lésions pulmonaires et pourcentage de poumon affecté calculé. Des analyses de corrélation ont démontré une corrélation positive entre la colonisation au sevrage et les 4 variables dépendantes indiquant une infection au moment de l’abattage : pointage moyen des lésions pulmonaires, pourcentage des poumons atteints, présence de M. hyopneumoniae sur l’épithélium bronchial et séroconversion. Ainsi, les stratégies visant à réduire la colonisation au moment du sevrage semblent être des éléments importants dans le contrôle global de M. hyopneumoniae dans les systèmes de production en ségrégation.
(Traduit par Docteur Serge Messier)
Introduction
Modern production systems have been developed to minimize the impact of respiratory diseases in swine operations. Segregated early weaning and all-in, all-out management programs have been designed to reduce both vertical and horizontal transmission of respiratory pathogens (1,2). However, despite the trend towards high health levels, Mycoplasma hyopneumoniae remains a significant pathogen in the pig industry (3). As a consequence of these production modifications, clinical signs present in the late finishing period instead of in the typical late nursery period (4), perhaps owing to a reduced prevalence of infection at weaning, lowered infection pressure in the nursery, and thus delayed clinical presentation (5). Late presentation, along with infection by other primary or secondary respiratory pathogens, has been associated with high morbidity, moderate mortality, and significant economic losses in affected populations (4,6).
There appears to be considerable variation in the severity of clinical signs between weekly production groups in offsite weaning herds, some groups reaching slaughter without obvious evidence of late M. hyopneumoniae infection and others showing clinical signs and lesions of varying degrees. It has been suggested that these differences may reflect the prevalence of infection at weaning, since the piglets presumably constitute the main infection source for the group (6). This hypothesis is based on the slow spread of M. hyopneumoniae, particularly when microbial pressure is low (7,8), as well as the apparent need to have a significant infected population within the group before clinical signs become evident and characteristic lesions can be detected (5).
Sow-to-piglet transmission is a way by which M. hyopneumoniae is maintained within swine populations (2,3). However, little information on sow-to-piglet transmission is available. It can be assumed that prevalence at weaning is a consequence of the rate of vertical transmission, which may be influenced by sow parity (3): an early work demonstrated that older sows were less likely to transmit M. hyopneumoniae to their offspring (9). Another influence might be infection pressure in the sow herd. These 2 factors vary in each farrowing group, resulting in fluctuations of the vertical transmission rate and the prevalence of infection among piglets at weaning.
A recent study of the effect of sow vaccination before farrowing on the vertical transmission rate showed that this intervention significantly reduced piglet colonization (6). Another study, evaluating the effect of sow antibiotic medication during lactation, showed a similar reduction in the prevalence of M. hyopneumoniae at weaning (10). Both studies suggested that colonization at weaning is the result of vertical transmission.
No previous studies had tested the hypothesis that M. hyopneumoniae colonization at weaning plays an important role in the clinical manifestation of late mycoplasmosis. Also, there had been no previous estimates of the effect of piglet colonization at weaning on the severity of disease for the group. Information was therefore needed to examine the concept that interventions focused on the weaning period constitute a useful approach in controlling the disease in growing populations.
The general objective of this study was to determine whether colonization at weaning was associated with the severity of respiratory disease due to M. hyopneumoniae in growing pigs. The specific aims of the study were to determine the relationships between the nasal presence of M. hyopneumoniae at weaning and 1) the frequency and severity of pneumonic lesions related to this agent at slaughter, 2) the presence of this agent at slaughter as identified from bronchial samples by polymerase chain reaction (PCR), and 3) seroconversion at the end of the growing period.
Materials and methods
Farm selection and vaccination protocol
Three farms of 3000 sows each were selected on the basis of an average prevalence of M. hyopneumoniae at weaning higher than 5%, as determined before the study by testing nasal swabs from a random sample of 30 piglets with nested PCR. The herds, designated as sow herds A, B, and C, had a prevalence at weaning of 33.3%, 23.3%, and 10.0%, respectively. In addition, these farms had a history of clinical signs and lung lesions associated with this microorganism, such as coughing in the late finishing stage, cranial-ventral lesions of pneumonia at slaughter, and seroconversion (3). All 3 herds belonged to the same company and were subject to the same nutritional, genetic, and general management procedures. The selected farms were multisite facilities, with offsite weaning and all-in, all-out flow in the nursery and finishing facilities. Most nurseries and finishers used mechanical ventilation of air at recommended industry standards. The selected herds were positive for Porcine reproductive and respiratory syndrome virus (PRRSV) at the time of the study, most farms showing signs at mid-nursery or early finishing. The gilts were vaccinated against M. hyopneumoniae with 2 doses of RespiSure (Pfizer Animal Health, Exton, Pennsylvania, USA) before introduction into the sow herd, at 90 and 120 d of age, and after introduction, at 160 d of age; gestating sows were not vaccinated against this organism. No medication directed to M. hyopneumoniae control was used in these sow herds. Piglets were weaned at an average of 19 d of age. Growing pigs were vaccinated with 2 doses of RespiSure at 4 and 6 wk of age. Medication for weaned pigs consisted of carbadox (Mecadox; Phibro Animal Health, Ridgefield Park, New Jersey, USA).
Study design
To achieve the established goals, 20 production groups from the selected sow herds were included, each group representing a different week in production and averaging 1200 pigs. Sample size calculations were based on expected prevalence parameters, an expected coefficient of correlation of 0.5, α-value of 0.05, and power of 0.80. The initial and final prevalence of M. hyopneumoniae infection was measured for each group to determine the correlation.
Prevalence at weaning (initial prevalence)
The initial prevalence (colonization) with M. hyopneumoniae was established by detection of the microorganism in nasal swabs of piglets 1 d before weaning. For sample size calculation, we used a 95% confidence level and an expected prevalence of 10% (11); we also assumed some losses. The calculated sample size was 39 piglets for each group under study. Therefore, 39 litters were randomly selected, including sows of all parities present in the farrowing group, and 1 piglet was randomly selected from each litter and ear-tagged. The nasal swabs, obtained with the Mini-Tip BD Culturette (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA), were tested for M. hyopneumoniae DNA by a nested-PCR technique previously described (12) and validated under both field and experimental conditions (13,14). Special care was taken to obtain samples at the same nasal depth (3.0 cm) and to avoid cross-contamination between piglets (6). In total, 780 piglets were tested.
Final status
To determine the final M. hyopneumoniae status for each group, the pigs were followed until slaughter (at an average age for the 20 groups of 185 d), when the severity of infection was assessed from the frequency and extent of lesions suggesting M. hyopneumoniae infection (3), organism identification in the bronchi (8,12), and seroconversion (15). Tagged pigs (the same animals as used for the determination of initial prevalence) were sent to the slaughterhouse in the same shipment so that the final assessment for each group could be performed in a single visit; the minimum sample size at the slaughterhouse was 30. Lungs were labeled according to the ear-tag number. At the slaughterhouse, the average lung lesion score was calculated as described previously (16), and the percentage of affected lungs was also determined. Bronchial swabs were taken from each labeled lung and tested by the same nested-PCR technique. In the chronic phase of the disease, bronchial sampling is more sensitive than nasal sampling for detecting M. hyopneumoniae and is therefore the preferred method (14,17). Precautions were taken at the time of sample collection to avoid cross-contamination between lungs: all material was decontaminated, and gloves were changed between samplings. Blood samples were also collected; the serum was tested for M. hyopneumoniae antibodies with the Tween 20 enzyme-linked immunosorbent assay (18). An adjusted optical density (ADJ OD) of 0.24 was considered a positive result (19). Seroprevalence and average ADJ OD values were then calculated.
Throughout the study, the pigs were handled and cared for according to an approved University of Minnesota Institutional Animal Care and Use Committee protocol.
Statistical analysis
A Spearman rank correlation analysis was performed to determine the relationship between the initial prevalence of M. hyopneumoniae (determined from nasal swabs at weaning; independent variable) and each of the dependent variables (performed on 1 set of numbers in separate models): average lung lesion score, percentage of affected lungs, final prevalence of M. hyopneumoniae infection (determined from bronchial samples at slaughter), and seroconversion. Owing to the lack of normal distribution of the data for the independent variable and the percentage of affected lungs, we used a nonparametric correlation analysis of that relationship. The r2 coefficient was calculated for each tested association; a value greater than 0.50 was considered to indicate a strong association between variables.
Results
The prevalence values at weaning varied widely between the groups from the 3 sow herds (Table I), even between consecutive groups from the same herd. Sow herd A showed the widest range of prevalence values, from 5.12% to 51.28%, and sow herd C the lowest values and lowest range, from 2.5% to 5.12%; the values in sow herd B ranged between 0.0% and 38.46%. Similarly, the results for the 4 variables assessed to determine the final M. hyopneumoniae status showed a wide range (Table II). Groups 13 and 14 were eliminated from the study for marketing reasons.
Table I.
Prevalence of Mycoplasma hyopneumoniae colonization at weaning (initial prevalence)
| Group | Sow herd (source) | Prevalence at weaning (%) |
|---|---|---|
| 1 | A | 51.28 |
| 2 | A | 28.2 |
| 3 | A | 48.7 |
| 4 | A | 30.76 |
| 5 | A | 17.94 |
| 6 | A | 5.12 |
| 7 | A | 15.38 |
| 8 | A | 17.94 |
| 9 | A | 15.78 |
| 10 | B | 28.2 |
| 11 | B | 38.46 |
| 12 | B | 7.69 |
| 13 | B | 2.56 |
| 14 | B | 0 |
| 15 | B | 7.69 |
| 16 | B | 5.12 |
| 17 | C | 5.12 |
| 18 | C | 2.5 |
| 19 | C | 5.12 |
| 20 | C | 2.56 |
Table II.
Evidence of M. hyopneumoniae in the different groups at slaughter
| Groupa | Average lung lesion score | Frequency of affected lungsb (%) | Frequency of DNA detectionc (%) | Average adjusted optical density of antibodiesd |
|---|---|---|---|---|
| 1 | 12.56 | 83.0 | 100 | 0.685 |
| 2 | 1.30 | 53.3 | 79.3 | 0.216 |
| 3 | 5.70 | 74.0 | 100 | 0.623 |
| 4 | 4.45 | 70.0 | 100 | 0.563 |
| 5 | 5.51 | 81.5 | 100 | 0.476 |
| 6 | 1.07 | 39.3 | 10.7 | 0.122 |
| 7 | 4.27 | 55.2 | 100 | 0.443 |
| 8 | 7.36 | 80.0 | 100 | 0.444 |
| 9 | 3.04 | 52.0 | 86.2 | 0.390 |
| 10 | 8.06 | 86.6 | 100 | 0.284 |
| 11 | 7.30 | 80.0 | 71.8 | 0.940 |
| 12 | 2.38 | 44.0 | 15.0 | 0.192 |
| 15 | 1.15 | 34.4 | 25.0 | 0.117 |
| 16 | 7.06 | 63.3 | 48.3 | 0.442 |
| 17 | 0 | 0 | 35.7 | 0.427 |
| 18 | 0.23 | 6.6 | 33.3 | 0.275 |
| 19 | 1.06 | 18.8 | 46.0 | 0.229 |
| 20 | 1.81 | 40.1 | 40.9 | 0.232 |
| Mean | 4.12 | 53.5 | 66.2 | 0.394 |
| Standard error | 0.80 | 6.3 | 8.0 | 0.050 |
Groups 13 and 14 were eliminated from the study for marketing reasons.
Lungs with lesions associated with M. hyopneumoniae infection.
In bronchial swabs by nested polymerase chain reaction.
In an enzyme-linked immunosorbent assay, a value of 0.24 being considered evidence of seroconversion.
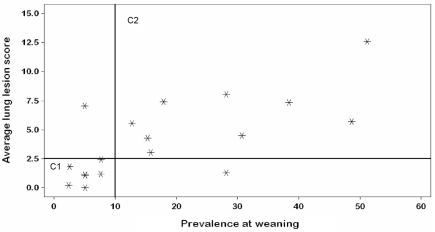
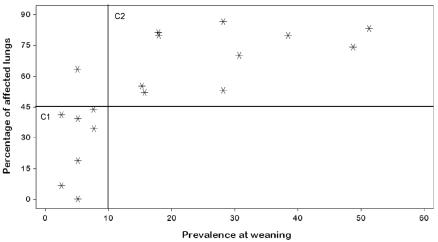
The correlation assessment showed evidence of a strong association between prevalence at weaning and all 4 dependent variables (Table III). Additionally, we observed clusters of study animals. With regard to average lung lesion score at slaughter (Figure 1), the 1st cluster consisted of the groups with a prevalence at weaning lower than 10% and a low lesion score, less than 2.5%; the 2nd cluster consisted of the groups with a prevalence at weaning higher than 10% and a higher lesion score. Similar clustering was observed with regard to the percentage of affected lungs at slaughter (Figure 2).
Table III.
Correlation between the prevalence of M. hyopneumoniae colonization at weaning (independent variable) and evidence of infection at slaughter (dependent variables)
| Dependent variable | r2 | P-value |
|---|---|---|
| Average lung lesion score | 0.5304 | 0.0009 |
| Percentage of affected lungs | 0.6448a | 0.0001a |
| Detection of DNA in bronchi | 0.5455 | 0.0007 |
| Seroconversion | 0.5079 | 0.0009 |
By nonparametric correlation analysis (Spearman rank correlations).
Figure 1.
Correlation of the presence of Mycoplasma hyopneumoniae in nasal swabs at weaning (independent variable) with the average lung lesion score (dependent variable); r2 = 0.5304, P = 0.0009. C1 — cluster 1; C2 — cluster 2.
Figure 2.
Correlation of the presence of M. hyopneumoniae in nasal swabs at weaning (independent variable) with the percentage of affected lungs (dependent variable); r2 = 0.6448, P = 0.0001.
Discussion
This study evaluated the association between the prevalence of M. hyopneumoniae colonization in piglets at weaning (initial prevalence) and the severity of M. hyopneumoniae infection at slaughter. The hypothesis was that the colonization prevalence among piglets in off-site weaning systems has a significant effect on the M. hyopneumoniae status of growing pigs. Under the conditions of this study, the results supported this hypothesis, demonstrating that initial prevalence can predict the clinical course of the disease with subsequent group segregation. This correlation was consistently observed for the 4 variables used to assess the final M. hyopneumoniae status: pneumonic lesions at slaughter (frequency and extent), colonization in the bronchi, and seroconversion. Therefore, the study has proven that, in addition to factors such as differences in housing, management (2,20), M. hyopneumoniae strain (21), and type of production system (4), the initial prevalence of colonization in the population is central in defining the clinical course of the disease. The results also suggest that a cut-off point in the prevalence at weaning could be considered as a predictor of subsequent disease severity. However, to determine the calculation and applicability of this value, more information is needed from experimental or controlled studies that would consider factors such as infection pressure in the sow herd, production system, intervention tools used against M. hyopneumoniae infection, and the role of other pathogens.
In this study, pneumonic lesions suggestive of M. hyopneumoniae infection in marketed animals were an important element in associating prevalence of colonization at weaning with subsequent disease severity. However, previous articles had suggested that lesions seen at slaughter are poor indicators of the lifetime prevalence of pneumonia (22,23). This previous information was derived from studies performed in farms with conventional farrow-to-finish systems, where clinical manifestation of the disease is found late in the nursery period (24). In these systems there is considerable time between infection and slaughter, so that many animals recover from the pneumonia, and lesions at slaughter do not reflect the extent of the problem during the animal’s life. In the present study, the animals were raised in segregated systems with all-in, all-out flow, where disease manifestation is delayed, pneumonia being seen late in the finishing period (4,6). Lung recovery may take 8 to 12 wk (25); therefore, it can be assumed that late-finishing pneumonia is more accurately detected at slaughter than is nursery pneumonia. In addition, both the average lung lesion score and the percentage of affected lungs were strongly and positively correlated with the presence of microbial DNA in bronchial swabs, demonstrating the role of M. hyopneumoniae in the lung lesions. A previous study (26) also showed an association between the severity of lung lesions and the identification of M. hyopneumoniae in bronchial samples by PCR.
The observed variation in the prevalence of M. hyopneumoniae colonization at weaning in groups from the same sow herd might reflect the fact that sows in the studied herds were not vaccinated before farrowing: rather, vaccination was performed exclusively during the acclimatization process (at 90, 120, and 160 d of age), which may have resulted in a high variability of shedding between animals. This is probably a frequent situation in sow herds in which M. hyopneumoniae infection is persistent (6,8).
Even with vaccination against M. hyopneumoniae, the groups with a high prevalence of piglet colonization with this organism at weaning had clinical disease during finishing, which suggests partial protection or, in some cases, vaccine failure. Considering the high variability in prevalence at weaning between the groups, we can assume that the vaccination timing was not appropriate for all groups, specifically those with high initial prevalence. As described before (27), another factor to consider should be the role of PRRSV in vaccine efficacy, since all the selected herds were positive for PRRSV at the time of the study, and mild clinical signs of the syndrome were documented during the nursery period. Variability in the prevalence of colonization at weaning and the presence of other infections during the nursery period support the idea of focusing all intervention efforts on the sow herd and suckling piglets.
Limitations of this study such as the use of only 1 kind of production system and only 1 company must be considered when interpreting and applying the results. Off-site weaning and segregated systems are extensively used in North America. However, a wide variety of other production systems, including the conventional farrow-to-finish system, are also used. Therefore, further studies testing the same hypothesis in other production systems are needed to determine the applicability of the results.
In general, this study provided evidence that disease due to M. hyopneumoniae may be controlled by reducing the rate of sow-to-piglet transmission of the organism in the farrowing unit, specifically in offsite weaning herds with all-in, all-out production, where it is assumed that colonization at weaning represents the main source of infection. Control strategies focused on reducing such colonization seem to be important in the global control of disease due to M. hyopneumoniae, at least in production systems with strict segregation. Such strategies may also result in a significant reduction in the use of antibiotics, vaccines, and manipulations in growing pigs. Previous studies showed that reduction of the prevalence of colonization at weaning was achieved by interventions in farrowing sows (6,10). However, future studies will be necessary to better understand which interventions or strategies are the most efficacious at reducing the prevalence in different production scenarios.
To conclude, this study has demonstrated that the initial prevalence of piglet colonization in a group plays an important role in defining the intensity at which disease due to M. hyopneumoniae will be presented and therefore provides evidence that disease severity can be predicted by prevalence at weaning, at least in segregated systems.
Acknowledgment
Funding for this study was provided by the Minnesota Pork Board.
References
- 1.Harris LD, Alexander TJ. Methods of disease control. In: Straw B, D’Allaire S, Mengeline W, Taylor D, eds. Diseases of Swine, 8th ed. Ames, Iowa: Iowa State Univ Pr, 1999:1077–1078.
- 2.Clark LK, Scheidt AB, Armstrong CH. The effect of all-in/all-out management on pigs from a herd with enzootic pneumonia. Vet Med. 1991;86:946–951. [Google Scholar]
- 3.Ross RF. Mycoplasmal diseases. In: Straw B, D’Allaire S, Mengeline W, Taylor D, eds. Diseases of Swine, 8th ed. Ames, Iowa: Iowa State Univ Pr, 1999:495–510.
- 4.Sibila M, Calsamiglia M, Vidal D, et al. Dynamics of Mycoplasma hyopneumoniae infection in 12 farms with different production systems. Can J Vet Res. 2004;68:12–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Pijoan C. Pathogenesis of disease in SEW pigs. Pig News Inform. 1997;18:65–66. [Google Scholar]
- 6.Ruiz A, Utrera V, Pijoan C. Effect of Mycoplasma hyopneumoniae sow vaccination on piglet colonization at weaning. J Swine Health Prod. 2003;11:131–135. [Google Scholar]
- 7.Ruiz A, Galina L, Pijoan C. Mycoplasma hyopneumoniae colonization of pigs sired by different boars. Can J Vet Res. 2002;66:79–85. [PMC free article] [PubMed] [Google Scholar]
- 8.Fano E, Pijoan C, Dee S. Dynamics and persistence of Mycoplasma hyopneumoniae infection in pigs. Can J Vet Res. 2005;69:223–228. [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin RFW. The phenomenon of suppressed respiratory disease in the litter of older sows. Vet Rec. 1965;77:383–387. [PubMed] [Google Scholar]
- 10.Cardona A. Prevalence of Mycoplasma hyopneumoniae in Different Parity Sows: Effect of Sow Medication and Aerosol Movement [MSc dissertation]. St. Paul, Minnesota: University of Minnesota, 2003:52–66.
- 11.Cannon RM, Roe RT. Livestock Disease Surveys: a Field Manual for Veterinarians. Canberra: Australian Bureau of Animal Health, 1982:33.
- 12.Calsamiglia M, Pijoan C, Trigo A. Application of a nested polymerase chain reaction assay to detect Mycoplasma hyopneumoniae from nasal swabs. J Vet Diagn Invest. 1999;11:246–251. doi: 10.1177/104063879901100307. [DOI] [PubMed] [Google Scholar]
- 13.Sibila M, Calsamiglia M, Segales J, et al. Association between Mycoplasma hyopneumoniae at different respiratory sites and presence of histopathological lung lesions. Vet Rec. 2004;155:57–58. doi: 10.1136/vr.155.2.57. [DOI] [PubMed] [Google Scholar]
- 14.Fano E, Pijoan C, Dee S. Evaluation of a nested-PCR technique from nasal samples to identify Mycoplasma hyopneumoniae in live animals. In: Proceedings of the 85th Conference of Research Workers in Animal Diseases (CRAWD); 2004 Nov 14–16; Chicago, Illinois:140.
- 15.Erlandson KR, Evans RB, Thacker BJ, et al. Evaluation of three serum antibody enzyme-linked immunosorbent assays for Mycoplasma hyopneumoniae. J Swine Health Prod. 2005;13:198–203. [Google Scholar]
- 16.Pointon AM, Davies PR, Bahnson PB. Disease surveillance at slaughter. In: Straw B, D’Allaire S, Mengeline W, Taylor D, eds. Diseases of Swine, 8th ed. Ames, Iowa: Iowa State Univ Pr, 1999:1111–1132.
- 17.Kurth KT, Hsu T, Snook ER, et al. Use of a Mycoplasma hyopneumoniae nested polymerase chain reaction test to determine the optimal sampling sites in swine. J Vet Diagn Invest. 2002;14:463–469. doi: 10.1177/104063870201400603. [DOI] [PubMed] [Google Scholar]
- 18.Bereiter M, Young TF, Joo HS, et al. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Microbiol. 1990;25:177–192. doi: 10.1016/0378-1135(90)90075-7. [DOI] [PubMed] [Google Scholar]
- 19.Thacker E. Mycoplasma hyopneumoniae ELISA cut point. J Swine Health Prod. 2003;11:220. [Google Scholar]
- 20.Done S. Enzootic pneumonia (mycoplasmosis) revisited. J Pig Vet Soc. 1996;38:40–61. [Google Scholar]
- 21.Vicca J, Maes D, Thermote L, et al. Patterns of Mycoplasma hyopneumoniae infection in Belgian farrow to finish pig herds with diverging disease course. J Vet Med. 2002;49:349–353. doi: 10.1046/j.1439-0450.2002.00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Noyes EP, Feeney DA, Pijoan C. Comparison of the effect of pneumonia detected during lifetime with pneumonia detected at slaughter on growth in swine. J Am Vet Med Assoc. 1990;197:1025–1029. [PubMed] [Google Scholar]
- 23.Sitjar M, Noyes EP, Simon X, et al. Relationships among sero-conversion to Mycoplasma hyopneumoniae, lung lesions, and production parameters in pigs. J Swine Health Prod. 1996;4:273–277. [Google Scholar]
- 24.Taylor DJ. Mycoplasma infections. In: Taylor DJ, ed. Pig Diseases, 7th ed. Cambridge, England: St. Edmundsbury Press, 1999: 180–190.
- 25.Sorensen V, Ahrens P, Barfod K, et al. Mycoplasma hyopneumoniae infection in pigs: duration of the disease and evaluation of four diagnostic assays. Vet Microbiol. 1997;54:23–34. doi: 10.1016/s0378-1135(96)01266-7. [DOI] [PubMed] [Google Scholar]
- 26.Calsamiglia M, Collins JE, Pijoan C. Correlation between the presence of enzootic pneumonia lesions and detection of Mycoplasma hyopneumoniae in bronchial swabs by PCR. Vet Microbiol. 2000;76:299–303. doi: 10.1016/s0378-1135(00)00245-5. [DOI] [PubMed] [Google Scholar]
- 27.Thacker EL, Thacker B, Young JTF, Halbur P. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000;8:1244–1252. doi: 10.1016/s0264-410x(99)00395-3. [DOI] [PubMed] [Google Scholar]