Abstract
The purpose of this study was to determine if ginseng fed at low levels enhances a horse’s antibody response to vaccination against Equid herpesvirus 1 (EHV-1). For 28 d, 5 horses received ground, powdered ginseng (35 mg/kg body weight, 1.7 mg/kg total ginsenosides) in molasses as a carrier, and 5 received molasses only. On day 14, each horse was vaccinated against EHV-1. The time course of the antibody response to vaccination was significantly altered in the horses receiving ginseng, a clinically relevant increase in antibody titer being observed by postvaccination day 2 compared with day 6 in the control horses. The horses receiving ginseng also had a significant decrease in serum levels of sodium and a significant increase in serum levels of potassium. No adverse effects of ginseng treatment were identified by hematologic and blood biochemistry profiles. Thus, low-dose dietary supplementation with ginseng in healthy horses may be a useful adjunct to vaccination.
Résumé
L’objectif de la présente étude était de déterminer si le ginseng nourri à petites doses augmente la production d’anticorps induits par la vaccination chez un cheval immunisé contre l’herpèsvirus équin 1 (EHV-1). Pendant 28 jours, 5 chevaux ont reçu du ginseng moulu (35 mg/kg de poids corporel; 1,7 mg/kg de ginsenosides total) dans de la mélasse comme véhiculant, et 5 ont reçu uniquement de la mélasse. Au jour 14, chaque cheval a été vacciné contre le EHV-1. La courbe de production d’anticorps suite à la vaccination était modifiée de manière significative chez les chevaux recevant du ginseng, une augmentation cliniquement significative du titre d’anticorps étant observée au jour 2 post-vaccination comparativement au jour 6 chez les chevaux témoins. Les chevaux recevant du ginseng montraient également une réduction significative des niveaux sériques de sodium et une augmentation significative des niveaux sériques de potassium. Aucun effet adverse du traitement au ginseng n’a été identifié par les profils hématologiques et biochimiques sanguins. Ainsi, un supplément alimentaire à faible dose avec du ginseng chez des chevaux en santé pourrait être un ajout utile à la vaccination.
(Traduit par Docteur Serge Messier)
Introduction
Equid herpesvirus 1 (EHV-1) and Equid herpesvirus 4 (EHV-4) are among the most prevalent viruses isolated from horse populations in many countries, including Canada (1), the United States (2), Australia (3), and France (4). Both EHV-1 and EHV-4 are associated with respiratory disease (5,6), but EHV-1 is also associated with abortion (7), neurologic disease (8), and neonatal foal disease (9). Horses infected with EHV-1 produce antibodies that target the virion membrane surface glycoproteins (10) and cross-react at least partially with EHV-4 (6). However, as a viral infection progresses through intermittent intracellular and extracellular phases, the ability of antibodies to recognize the cell-surface antigens is restricted to the extracellular phase. For this reason, immunologic experience provides limited protection (5). Vaccination against the disease is routinely performed, but despite the customary presence of adjuvants in commercially available vaccines, EHV-1 vaccines typically produce a poor antibody response and provide incomplete protection against infection (2). Augmenting the serologic response to an adjuvanted EHV-1 vaccine may improve clinical protection against infection in horses (11).
It has been reported that ginseng behaves synergistically with other adjuvants to improve the immunologic response to vaccination (12). Ginseng contains a number of bioactive saponins (ginsenosides) that have been associated with increased antibody responses to vaccination in guinea pigs (12), pigs (13), mice (14,15), and dairy cattle (16); each study used parenteral injection of ginseng, ginseng extract, or both. Although the practice shows considerable promise, this method of administration is impractical for people working with horses in the field. One study has reported successful potentiation of influenza vaccination in human subjects by dietary administration of ginseng, 1.4 mg/kg (17). We hypothesized that feeding ginseng at an economically feasible dose would increase the serologic response to EHV-1 vaccination in immunologically experienced horses.
Materials and methods
Horses and experimental design
Ten healthy mature mixed-breed horses (3 mares, 7 geldings) that had previously received EHV-1 vaccinations as recommended were housed in a research barn at the University of Guelph, Guelph, Ontario. The horses received a balanced ration that met their nutritional requirements (18) and consisted of 1.5 kg of concentrate pellets and one-third of a bale of dry hay twice daily. The concentrate was composed of grain corn (41%), barley and oats (28% each), 1.6% Shur-Gain premix (1.6%; Shur Gain, St. Marys, Ontario), dry molasses and animal–vegetable (A–V) fat blend (0.5% each), trace mineral salt (0.2%), dicalcium phosphate (0.1%), and vitamin E/selenium and magnesium oxide (0.05% each). Additional trace minerals (magnesium, copper, iodine, and cobalt, at concentrations of 1200, 330, 70, and 40 mg/kg, respectively), in a block containing 95.6% sodium chloride, and water were provided ad libitum. The horses received daily turnout into small paddocks and were housed indoors on straw at night.
Five horses (Table I) were randomly selected to receive ground air-dried ginseng (Rainey Ginseng Farms, Delhi, Ontario), 35 mg/kg body weight (BW) in a carrier of molasses and bread, as a bolus in their morning grain ration for 28 d. The remaining 5 horses received the carrier alone and served as controls. On day 14 all horses received an intramuscular injection of 2 mL of Pneumabort-K + 1b (Ayerst, Guelph), a killed virus vaccine against EHV-1. On days 0, 7, and 14 (before vaccination), days 15 through 21, and day 28, venous blood was collected by jugular puncture into silicone-coated, heparin-containing Vacutainer tubes (Fisher Scientific, Nepean, Ontario).
Table I.
Details for the horses receiving dietary ginseng, 35 mg/kg body weight (BW) daily, in a molasses carrier and control horses receiving only the carrier
| Horse no. | Sex | Age (y) | BW (kg) | Ginseng dose (g/d) |
|---|---|---|---|---|
| 1 | F | 12 | 547 | 19.1 |
| 2 | M | 9 | 626 | 0 |
| 3 | F | 6 | 513 | 0 |
| 4 | M | 22 | 463 | 16.2 |
| 5 | M | 5 | 417 | 0 |
| 6 | F | 14 | 438 | 15.3 |
| 7 | M | 7 | 405 | 14.2 |
| 8 | M | 11 | 420 | 0 |
| 9 | M | 22 | 454 | 15.9 |
| 10 | M | 12 | 612 | 0 |
The protocol for animal care and use was approved by the Equine Research Centre (University of Guelph) Animal Care Committee and was in accordance with the guidelines of the Canadian Council on Animal Care (19).
Antibody analysis
At Cornell University Diagnostic Laboratory (Ithica, New York, USA), the total serum concentration of IgG against EHV-1 in all the samples of jugular venous blood was determined by serum neutralization (20). All tests were conducted in duplicate, starting at a dilution of 1:4 and progressing in 2-fold dilutions.
T-lymphocyte analysis
The venous blood samples collected on days 0, 7, 14, 21, and 28 were analyzed by single-color flow cytometry at Princess Margaret Hospital (Toronto, Ontario) for total T-lymphocytes and lymphocytes expressing cell-surface markers CD4 and CD8 as percentages of the total peripheral blood leukocyte (PBL) count. Aliquots (100 μL) were removed from the samples for indirect labeling of cell-surface antigens by means of fluorescent antibodies (Serotec, Raleigh, North Carolina, USA), which were titrated and used at optimal concentrations for flow cytometric analysis. The PBLs from each sample were incubated for 20 min at room temperature with antibodies against equine immunoglobulin, CD8, CD4, or CD2 raised in mice or, in the case of CD2, rats.
The cytometric analyses were performed with the use of a Becton Dickinson FACScan flow cytometer (Mountain View, California, USA). Gates were defined around the lymphocyte populations by forward and side-angle light-scatter characteristics. Unlabeled equine PBLs and equine PBLs treated in parallel with isotype- control antibodies (Rhodophyta phycoerythrin-conjugated rat IgG2a or fluorescein isothiocyanate-conjugated mouse IgG2a, IgG2b, or IgG1; all from Serotec) were used to define background values and autofluorescence. At least 10 000 events in the defined lymphocyte gate were analyzed for each sample, with the use of CellQuest software (Becton Dickinson).
Blood biochemistry and hematologic analysis
The venous blood samples collected on days 0, 14, and 28 were also analyzed at VitaTech Veterinary Research Laboratories (Markham, Ontario), by means of a Hitachi 911 Biochemical Analyser (Boehringer Mannheim, Laval, Quebec), for albumin/globulin ratio and for concentrations of albumin, alkaline phosphatase, urea, calcium, chloride, cholesterol, creatine phosphokinase, creatinine, glucose, phosphorus, potassium, total protein, aspartate aminotransferase, and sodium. Counts of leukocytes, erythrocytes, neutrophils, lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells, along with the hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, were obtained with the use of an Advia 120 (Bayer Corporation, Etobicoke, Ontario).
Ginsenoside analysis
Quantitative determination of total and individual ginsenosides in the powdered ginseng was conducted by high-performance liquid chromatography (Beckman Instruments, Fullerton, California, USA) at the Ottawa-Carleton Institute of Biology, University of Ottawa, Ottawa, Ontario, as previously described (21). Peak identities in the samples were confirmed by relative retention time and ultraviolet spectral analysis in comparisons with injections of pure ginsenosides Rg1, Re (University of Chicago, Chicago, Illinois, USA), Rf, Rb1, Rc, Rb2, and Rd (Indofine Chemical Company, Belle Mead, New Jersey, USA).
Data analysis
A log10 transformation was performed on all the antibody-titer data. Data for each horse were then converted to the change from baseline, and the mean change from baseline for each group of horses was calculated. Antibody results are presented as mean log10 changes from baseline, lymphocyte data as percentages of the total PBL count, and all other data as means, with the standard error of the mean in parenthesis. The antibody data were analyzed with a 1-tailed, 1-way repeated-measures analysis of variance (ANOVA) on transformed data; all other data were analyzed with a 2-tailed, 1-way repeated-measures ANOVA. Statistical significance was accepted at P ≤ 0.05.
Results
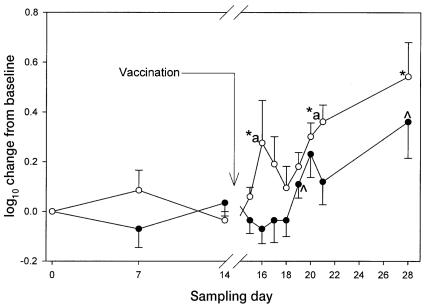
One horse in each group did not respond to EHV-1 vaccination with respect to the titer of antibody against EHV-1. However, because their expression of CD4 and CD8 increased significantly, their data were included in the analysis. As a group, the control horses had a significant antibody response (P < 0.05) to the vaccine, with an initial peak change from baseline on day 20 of 0.2310 (0.093). By day 21 the antibody levels had fallen almost to baseline, but there was a significant peak change (P < 0.05) again, on day 28, at 0.3610 (0.145). The time course of the antibody response was significantly altered by supplementation with ginseng (Figure 1). The initial significant peak change (P < 0.05) was earlier, on day 16 (0.276 [0.171]), and a 2nd significant peak (P < 0.05) was observed on day 21 (0.3610 [0.067]) and a final significant peak (P < 0.05) at day 28 (0.5420 [0.137]). The maximum change in antibody titer was observed on day 28 in both groups, the change for the control horses being about 55% of that of the horses that were fed ginseng. The difference in antibody response between the 2 groups was significant on days 16 and 21.
Figure 1.
Changes in antibody titer from baseline (mean and standard error) after vaccination (on day 14, after sampling) against Equid herpesvirus 1 (EHV-1) in 5 control horses (black circles) and 5 horses fed ginseng (white circles). Significant increases (P < 0.05) for the ginseng-treated horses are denoted by a star, for the control horses by a caret, and between the 2 groups by an “a”.
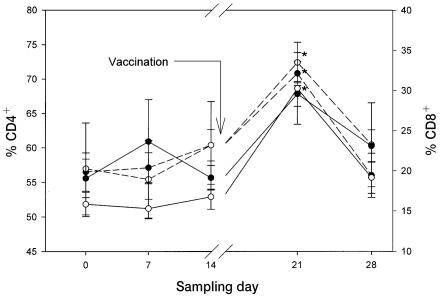
Vaccination resulted in a significant increase (P < 0.05) in the percentage of plasma T-lymphocytes among all PBLs on days 21 and 28, to 80.4% (4.7%) and 84.4% (3.6%), respectively, in the control horses; significant increases were not observed in the horses fed ginseng. On day 21, the percentage of PBLs expressing CD4 was significantly (P < 0.05) and similarly increased in the 2 groups, at 70.8% (1.8%) in the control group and 72.4% (3.0%) in the group fed ginseng, whereas the percentage expressing CD8 was significantly increased (P < 0.05) only in the group fed ginseng, at 30.2% (4.5%) (Figure 2).
Figure 2.
Percentages of total peripheral blood leukocytes expressing CD4 (dashed lines) and CD8 (solid lines) cell-surface markers in the same 2 groups of horses. Significant differences from baseline (P < 0.05) are denoted by a star.
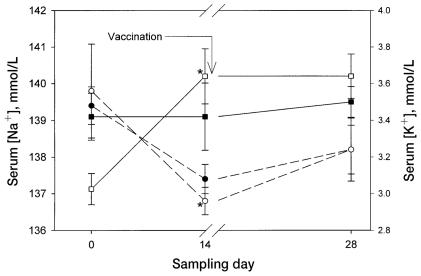
On day 14, the serum sodium concentration was lower than at baseline in both groups of horses (Figure 3), but significantly so only in the ginseng-treated horses (P < 0.05); there was no significant difference between the groups. By the same day, the serum potassium concentration had significantly decreased (P < 0.05), from 3.0 (0.1) to 3.6 (0.1) mmol/L, in the ginseng-treated horses. On day 0, the serum potassium concentration had been significantly lower in the ginseng-treated horses than in the control horses. No other changes in blood biochemistry profile were identified in either group. There were no significant differences between the 2 groups in hematologic profile.
Figure 3.
Changes in serum concentrations of sodium (dashed lines) and potassium (solid lines) in the same 2 groups of horses. Significant differences from baseline (P < 0.05) are denoted by a star.
The total ginsenoside contribution to the dry weight of the product fed to the horses was 5.889%, of which Rb1, Re, Rd, Rc, Rg1, and Rb2 accounted for 61%, 26%, 6%, 4%, 2%, and 0.6%, respectively; Rb1 accounted for approximately 3.5% of the dry weight of the product and Re for approximately 1.5%. No Rf was detected in the ginseng used in this study.
Discussion
Our study has demonstrated that dietary ginseng, at a daily dose of 35 mg/kg BW for 2 wk before and 2 wk after parenteral EHV-1 vaccination, significantly improves the early time course and magnitude of the antibody response in horses, in addition to influencing the serum electrolyte balance.
The only study to previously investigate the adjuvant effect of dietary ginseng was that by Scaglione et al (17), who reported efficacy at a dose of 1.4 mg/kg in humans. The rationale for our higher dosage follows from the fact that Scaglione et al used a proprietary extract (Ginsana 115) standardized to 4% total ginsenosides and did not report the relative proportions of each ginsenoside. Individual ginsenosides have unique immunologic effects (12,14), and their bioavailability profiles differ significantly (22). One of the ginsenosides that is reportedly more potent as a vaccine adjuvant, Rb1 (14,16), has a very poor bioavailability, 4.4% (22), whereas Rg1, also an effective adjuvant (14), has a higher bioavailability, 18.4% (22). Unfortunately, these 2 ginsenosides are antagonists and partially inhibit each other, such that the adjuvant potential of their combination is lower than that of each individual ginsenoside (12). For this reason, the relative proportions that enter the circulation may be very important in determining bioactivity. The dominant ginsenoside in our ginseng product, accounting for 61% of the total ginsenoside content, was Rb1; only 2% was Rg1. Thus, although the total ginsenoside content of our sample was relatively high, at more than 5%, the bioavailability of the governing active principal would have been low. We therefore used a higher dose, which was still economical and practical for administration to horses in the field.
The improved serologic response to EHV-1 vaccination that we demonstrated was clinically relevant, in that the IgG titer was greater after vaccination in the ginseng-fed horses than in the controls. It is possible that a higher ginseng dose would have produced even greater effects, as some of the immunomodulatory effects of ginseng are reported to be dose-dependent (16,23). The mechanism of vaccine potentiation may involve the ability of ginseng to increase the production of cytokines, particularly interleukin 2 and interferon gamma (24). These cytokines play central roles in T-cell activation and migration (25), and they support B-cell differentiation and subsequent production of soluble antibody (26). The observed increase in the proportion of CD8+ cells in the ginseng-fed horses is not likely of clinical significance, as the magnitude of the increase was similar to that in the control horses.
The effect on serum electrolyte balance observed in our study may simply reflect the lower baseline serum potassium concentration in the ginseng-treated horses, which was, however, within the expected reference interval for each horse. If, indeed, the increase in serum potassium concentration in the ginseng-treated horses resulted from ginseng supplementation and was not some artifact of randomization, it may in part be explained within the context of muscle ion transport. It has been reported that Rb1 inhibits Na+/K+-ATPase (27), the ATP-dependent sodium–potassium pump, in vascular endothelial cells, which results in reduced Na+ efflux. Furthermore, other ginsenosides (Rg3 and Re) mobilize intracellular Ca2+, which increases the intracellular concentration of Ca2+ and opens Ca2+-dependent K+ channels, thus increasing the net K+ efflux (28). The resultant localized increases in the extracellullar K+ concentration have been shown to cause hyperpolarization of cardiomyocyte membranes, resulting in synthesis and release of the vasodilator nitric oxide. This may in part account for the putative cardiovascular benefits of consuming ginseng (29).
The minimum inhibitory concentration (MIC50) of Rb1 reported to influence voltage-gated channels is 6.3 μmol/L (27). With a molecular weight of 1109.5 (30), and assuming 4.4% bioavailability in a horse with approximately 300 L of total body water (31), Rb1 would have to be given at an oral dose of approximately 47 g/d to achieve the reported MIC50. Our horses were receiving only about 0.5 g of Rb1 daily, far below the dose that should be bioactive. The ginsenosides in our experiment may have acted synergistically on the Na+/K+-ATPase- and Ca2+-dependent K+ channels, whereas other reports have concerned primarily individual ginsenosides.
Our study did not identify any toxic effects of dietary supplementation with ginseng at a daily dose of 35 mg/kg BW for 28 d. We conclude that feeding ginseng at this dosage for 28 d is safe and may be a useful adjuvant to vaccination with EHV-1 in immunologically experienced horses. Further research into the adjuvant applications and characteristics of ginseng and its individual ginsenosides is warranted.
Acknowledgments
This research was funded by the Ontario Horse Racing Industry Association, the Canadian Ginseng Research Foundation, the Rural Job Strategy Fund, and Bioniche Life Sciences. We extend our gratitude to Dr. John Arnason for the kind gift of the ginseng phytochemical analysis and to Dr. Michael Lindinger for assistance in interpreting the biochemistry data.
References
- 1.Carman S, Rosendal S, Huber L, et al. Infectious agents in acute respiratory disease in horses in Ontario. J Vet Diagn Invest. 1997;9:17–23. doi: 10.1177/104063879700900104. [DOI] [PubMed] [Google Scholar]
- 2.Mumford EL, Traub-Dargatz JL, Carman J, et al. Occurrence of infectious upper respiratory tract disease and response to vaccination in horses on six sentinel premises in northern Colorado. Equine Vet J. 2003;35:72–77. doi: 10.2746/042516403775467379. [DOI] [PubMed] [Google Scholar]
- 3.Gilkerson J, Jorm LR, Love DN, Lawrence GL, Whalley JM. Epidemiological investigation of Equid herpesvirus-4 (EHV-4) excretion assessed by nasal swabs taken from thoroughbred foals. Vet Microbiol. 1994;39:275–283. doi: 10.1016/0378-1135(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 4.Taouji S, Collobert C, Gicquel B, et al. Detection and isolation of equine herpesviruses 1 and 4 from horses in Normandy: an autopsy study of tissue distribution in relation to vaccination status. J Vet Med. 2002;49:394–399. doi: 10.1046/j.1439-0450.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 5.van Maanen C. Equine herpesvirus 1 and 4: an update. Vet Q. 2002;24:58–78. [PubMed] [Google Scholar]
- 6.Ostlund EN. The equine herpesviruses. Vet Clin North Am Equine Pract. 1993;9:283–294. doi: 10.1016/s0749-0739(17)30396-6. [DOI] [PubMed] [Google Scholar]
- 7.Smith KC, Blunden AS, Whitwell KE, Dunn KA, Wales AD. A survey of equine abortion, stillbirth and neonatal death in the UK from 1988 to 1997. Equine Vet J. 2003;35:496–501. doi: 10.2746/042516403775600578. [DOI] [PubMed] [Google Scholar]
- 8.Cardwell J, Smith K, Newton R, Blunden T, Bestbier M, Whitwell K. EHV paralytic disease in the south of England. Vet Rec. 2003;152:441–442. [PubMed] [Google Scholar]
- 9.Bryans JT, Swerczek TW, Darlington RW, Crowe MW. Neonatal foal disease associated with perinatal infection by equine herpesvirus 1. J Equine Med Surg. 1977;1:20–26. [Google Scholar]
- 10.Allen GP, Yeargan MR. Use of lambda gt11 and monoclonal antibodies to map the genes for the six major glycoproteins of equine herpesvirus 1. J Virol. 1987;61:2454–2461. doi: 10.1128/jvi.61.8.2454-2461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluck R, Burri KG, Metcalfe I. Adjuvant and antigen delivery properties of virosomes. Curr Drug Deliv. 2005;2:395–400. doi: 10.2174/156720105774370302. [DOI] [PubMed] [Google Scholar]
- 12.Rivera E, Daggfeldt A, Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. 2003;91:19–27. doi: 10.1016/s0165-2427(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 13.Rivera E, Hu S, Concha C. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine. 2003;21:1149–1157. doi: 10.1016/s0264-410x(02)00518-2. [DOI] [PubMed] [Google Scholar]
- 14.Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng. Vaccine. 2005;23:5533–5542. doi: 10.1016/j.vaccine.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Sun HX, Ye YP, Pan HJ, Pan YJ. Adjuvant effect of Panax notoginseng saponins on the immune responses to ovalbumin in mice. Vaccine. 2004;22:3882–3889. doi: 10.1016/j.vaccine.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Concha C, Lin F, Persson Waller K. Adjuvant effect of ginseng extracts on the immune response to immunization against Staphylococcus aureus in dairy cattle. Vet Immunol Immunopathol. 2003;91:29–37. doi: 10.1016/s0165-2427(02)00264-7. [DOI] [PubMed] [Google Scholar]
- 17.Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardized ginseng extract G115 for potentiating vaccination against common cold and/or influenza syndrome. Drug Exp Clin Res. 1996;22:65–72. [PubMed] [Google Scholar]
- 18.Subcommittee on Horse Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient Requirements of Horses, 5th revised ed. Washington, DC: National Academy Press, 1989.
- 19.Olfert ED, Cross BM, McWilliams AA, eds. Guide to the Care and Use of Experimental Animals. Volume 1. Ottawa, Ontario: Canadian Council on Animal Care, 1993.
- 20.Gibson JS, O’Neill T, Thackray A, Hannant D, Field HJ. Serological responses of specific pathogen-free foals to equine herpesvirus-1: primary and secondary infection, and reactivation. Vet Microbiol. 1992;32:199–214. doi: 10.1016/0378-1135(92)90145-j. [DOI] [PubMed] [Google Scholar]
- 21.Assinewe VA, Baum BR, Gagnon D, Arnason JT. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agric Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- 22.Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 23.Jie YH, Cammisuli S, Baggiolini M. Immunomodulatory effects of Panax ginseng C.A. Meyer in the mouse. Agents Actions. 1984;15:386–391. doi: 10.1007/BF01972376. [DOI] [PubMed] [Google Scholar]
- 24.Liou CJ, Li ML, Tseng J. Intraperitoneal injection of ginseng extract enhances both immunoglobulin and cytokine production in mice. Am J Chin Med. 2004;32:75–88. doi: 10.1142/S0192415X04001771. [DOI] [PubMed] [Google Scholar]
- 25.Lipkowitz S, Greene WC, Rubin AL, Novogrodsky A, Stenzel KH. Expression of receptors for interleukin 2: role in the commitment of T lymphocytes to proliferate. J Immunol. 1984;132:31–37. [PubMed] [Google Scholar]
- 26.Chirmule N, Kalyanaraman VS, Lederman S, et al. HIV-gp 160-induced T cell-dependent B cell differentiation. Role of T cell–B cell activation molecule and IL-6. J Immunol. 1993;150:2478–2486. [PubMed] [Google Scholar]
- 27.Cao J, Zheng YQ, Liu TP, Feng LZ. Inhibitory effects of ginsenoside Rg1 and Rb1 on rat brain microsomal Na+, K(+)-ATPase activity. Zhongguo Yao Li Xue Bao. 1990;11:10–14. [PubMed] [Google Scholar]
- 28.Bai CX, Takahashi K, Masumiya H, Sawanobori T, Furukawa T. Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes. Br J Pharmacol. 2004;142:567–575. doi: 10.1038/sj.bjp.0705814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen CJ. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–192. [PubMed] [Google Scholar]
- 30.Zhang B, Hata R, Zhu P, et al. Prevention of ischemic neuronal death by intravenous infusion of a ginseng saponin, ginsenoside Rb(1), that upregulates Bcl-x(L) expression. J Cereb Blood Flow Metab. 2006;26:708–721. doi: 10.1038/sj.jcbfm.9600225. [DOI] [PubMed] [Google Scholar]
- 31.Forro M, Cieslar S, Ecker GL, Walzak A, Hahn J, Lindinger MI. Total body water and ECFV measured using bioelectrical impedance analysis and indicator dilution in horses. J Appl Physiol. 2000;89:663–671. doi: 10.1152/jappl.2000.89.2.663. [DOI] [PubMed] [Google Scholar]