Abstract
The presence of Bovine herpesvirus 4 (BoHV-4) was investigated by several methods in 24 aborted bovine fetuses. Polymerase chain reaction (PCR) and in situ DNA hybridization proved the presence of BoHV-4 DNA in 7 (29%) of the fetuses. The BoHV-4 genome was detected in the cytoplasm of splenic lymphocytes and monocytes, and sometimes in renal tubular epithelial cells or hepatic Kupffer cells, in all 7 PCR-positive fetuses. However, BoHV-4-specific monoclonal antibody failed to detect viral antigen in the formalin-fixed, paraffin-embedded tissue samples. No bacterial pathogens were found in the tissues of the BoHV-4-positive fetuses. Fungi were detected in 1 sample, and antibody to bovine viral diarrhea virus was detected in another. These results indicate that BoHV-4 could play a role in reproductive disorders of cattle, including abortion.
Résumé
La présence du herpesvirus bovin de type 4 (BoHV-4) a été investiguée par différentes méthodes chez 24 avortons bovins. L’amplification en chaîne par la polymérase (PCR) et l’hybridation in situ de l’ADN ont montré la présence d’ADN du BoHV-4 dans 7 fœtus (29 %). Le génome du BoHV-4 a été détecté dans le cytoplasme des lymphocytes et des monocytes de la rate, et quelquefois aussi dans les cellules épithéliales des tubules rénaux ou dans les cellules de Kupffer du foie, de tous les avortons positifs par épreuve PCR. Toutefois, des anticorps monoclonaux spécifiques au BoHV-4 n’ont pas permis de détecter d’antigènes viraux dans les échantillons de tissus fixés à la formaline et paraffinés. Aucun agent pathogène bactérien n’a été retrouvé dans les tissus des fœtus positifs pour BoHV-4. Des champignons ont été détectés dans un échantillon et des anticorps dirigés contre le virus de la diarrhée virale bovine dans un autre. Ces résultats indiquent que le BoHV-4 pourrait jouer un rôle dans les problèmes de reproduction et lors d’avortement chez les bovins.
(Traduit par Docteur Serge Messier)
Bovine herpesvirus 4 (BoHV-4), a member of the subfamily Gammaherpesvirinae and the genus Rhadinovirus, is found world wide in cattle populations. It has a 146-kb DNA genome and replicates in a wide variety of cell types of various species (1–3). The virus has been detected in various tissues of reproductive organs and has been isolated from aborted fetuses. Although epidemiologic and experimental data suggest its involvement, BoHV-4 has not been proven to be a causative agent in bovine reproductive disorders (4,5).
The α-herpesviruses Bovine herpesvirus 1 (BoHV-1), also known as infectious bovine rhinotracheitis virus (IBR virus), and Bovine herpes-virus 5, also known as bovine encephalitis virus, as well as BoHV-4, which is a γ-herpesvirus, have all been implicated in bovine abortion (6). In a 10-y survey (4), IBR virus was detected in 485 (5.4%) of 8962 specimens from bovine abortions and stillbirths, and BoHV-4 was isolated from 47 specimens (0.5%).
Postpartum metritis and mastitis are the clinical signs associated with BoHV-4. The virus has been identified and detected by several methods in tissue from cattle with endometritis (5). It was first isolated in uterine exudate from a cow with metritis (7). Fetuses inoculated with this strain at 3 or 4 mo of gestation died with lymphoreticular activation in the lungs and lymph nodes (8). Inclusion bodies typical for BoHV-4 were observed in various organs of 1 aborted fetus, but no attempt was made to isolate the virus (9). There are reports of isolation of BoHV-4 from aborted fetuses (4,10) and from infected tissues of the reproductive tract of cows with a high incidence of postpartum metritis, pustular vulvovaginitis, abortion, stillbirth, and infertility (8,11). A seroepidemiologic case–control study in the province of Liege, Belgium, found the seroprevalence of BoHV-4 infection to be significantly higher among cows with a history of abortion than in a randomly selected control group: 17.2% versus 10.0% (12). Pregnant rabbits evaluated as a laboratory model of BoHV-4 infection (13) had mild vulvovaginitis and endometritis after intravenous, intravaginal, and intrauterine administration of infective virus at midgestation, and intrauterine inoculation resulted in abortion; the virus was isolated from the fetuses, which indicates transplacental infection. Lesions of the placenta and virus isolation from the placenta were not reported.
This paper describes the presence of the BoHV-4 genome in tissues of 7 aborted bovine fetuses and provides data from polymerase chain reaction (PCR), in situ DNA hybridization (ISH), and immunohistochemistry (IHC) studies in the 7 cases.
In the period September 2002 to March 2005, 24 aborted bovine fetuses from 22 dairies in Hungary were sent to the Central Veterinary Institute, Budapest, Hungary, for diagnostic investigation. The organs underwent the same histologic, bacteriologic, and virus isolation studies, as well as nested PCR to detect BoHV-4 DNA. The liver, lungs, spleen, and kidneys of the 7 PCR-positive fetuses underwent ISH and IHC studies; in 5 cases lymph nodes were also tested.
The tissue samples from all 24 cases were fixed in 10% formalin and embedded in paraffin; 4-μm-wide sections were stained with hematoxylin–eosin (H–E). Liver and kidney were also stained with Warthin–Starry and Levaditi silver stains for detecting leptospires.
Samples of the stomach contents were cultured in parallel aerobically for 2 d at 37°C on blood and nutrient agar and anaerobically on blood agar for 6 d at 37°C in an atmosphere containing 10% CO2 for isolating Brucella. Smears of the stomach contents were stained by Stamp’s method for detecting bacteria and fungi. For virus isolation, tissues were homogenized in porcelain mortars in 0.5 mL of minimum essential medium (MEM). The supernatant was inoculated onto bovine arterial endothelial cell cultures with 1.5 g/L of streptomycin and 1.5 × 106 IU/L of penicillin. The culture medium was changed the next day, and the cultures were checked daily for 2 wk for any cytopathic effect.
Supernatants of homogenized spleen tissue that had undergone phenol-chloroform DNA extraction and proteinase K digestion (14) were analyzed by nested PCR assay specific for open reading frame (ORF) 20 of BoHV-4 (15); ORF 20 encodes a protein conserved in γ-herpesviruses. The assay is specific and able to amplify 0.2 plaque-forming units of the virus (16).
We used an IHC method to detect Chlamydia, Coxiella burnetii, Toxoplasma gondii, BoHV-1, and BoHV-4 in the formalin-fixed, paraffin- embedded tissue samples from the 7 PCR-positive cases. Briefly, after dewaxing the sections, we retrieved antigen by incubation with 0.1% protease XIV solution (Sigma-Aldrich, St. Louis, Missouri, USA) at 37°C for 10 min (for Chlamydia, T. gondii, and BoHV-1) or by heating in a microwave oven (750 W) for 20 min in citrate buffer (pH 6.0) (for C. burnetii). To detect BoHV-4, we treated 3 serial sections from every tissue sample with (a) 0.1% protease XIV solution at 37°C for 10 min, (b) heating in a microwave oven (750 W) for 20 min with citrate buffer (pH 6.0), or (c) nothing. Next, the sections were incubated in 3% H2O2 solution for 10 min and then in a 2% solution of skim milk powder for 20 min. The sections were incubated at 4°C overnight with primary antibodies, at the following dilutions: 1:100 Chlamydia lipopolysaccharide (clone AC-1; Progen GmbH, Heidelberg, Germany), 1:2000 C. burnetii (rabbit, kindly provided by Wolfgang Baumgärtner, Department of Pathology, University of Veterinary Medicine, Hannover, Germany), 1:200 T. gondii (rabbit; Biogenex, San Ramon, California, USA), 1:20 000 BoHV-1 (clone F2; VMRD, Pullman, Washington, USA), and 1:1000 BoHV-4 (clone VB MO88; Cypress Diagnostics, Langdorp, Belgium). Antibody binding was detected with a horseradish peroxidase (HRP)-labeled streptavidin–biotin kit (Universal LSAB2 Kit-HRP; DAKO, Glostrup, Denmark), used according to the manufacturer’s instructions. The sections were treated with 3-amino-9-ethylcarbazole solution (Sigma- Aldrich) containing 0.01% H2O2 at room temperature for 10 min, counterstained with Mayer’s hematoxylin for 20 s, and covered with glycerol gelatin. Tissue sections infected with Chlamydia, C. burnetii, T. gondii, or BoHV-1 and cell pellets infected with BoHV-4 were used as positive controls.
A sensitive nonradioactive ISH test was used to visualize BoHV-4 DNA in formalin-fixed, paraffin-embedded tissue sections. A sequence 567 base pairs (bp) long (nucleotide positions 27360 to 27927; GenBank accession number AF318573) in ORF 20 was amplified by PCR with digoxigenin (DIG)-labeled uridine triphosphate (DIG Probe Synthesis Kit; Roche, Basel, Switzerland). These DIG-labeled BoHV-4-specific DNA molecules were used as ISH probes and were detected with antibodies against DIG that were conjugated with HRP. The immunoreactive products were visualized with NBT/ BCIP (Roche). This method demonstrated excellent sensitivity: the ISH signal was clear and the background negligible (17). For negative controls, we used spleen samples that were BoHV-1-positive and BoHV-4-negative by PCR.
In only 3 fetuses were there histologic lesions, of mild purulent bronchitis in 1 fetus, mild subacute serous hepatitis in 2 fetuses, and mild serous lymphadenitis in 1 fetus. Bacteriologic investigations and virus isolation attempts gave negative results in all cases. Antibody to bovine viral diarrhea virus (BVDV) was detected in tissues from fetus 4, and Aspergillus fumigatus and Mucor spp. were detected in tissues from fetus 5; neither fetus had histologic lesions. Serum samples obtained from 4 of the 6 mothers gave negative results for B. abortus by an indirect enzyme-linked immunoassay, for C. burnetii antibodies by the complement fixation test, and for leptospiral antibodies by the microscopic agglutination test; however, 1 sample contained chlamydial complement-fixing antibody, at a titre of 1:20.
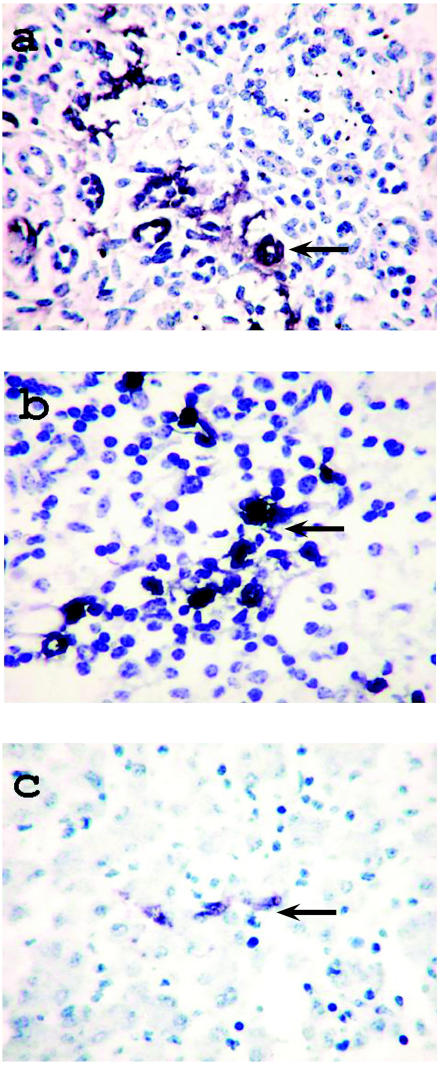
Although IHC testing did not detect Chlamydia, C. burnetii, T. gondii, BoHV-1, or BoHV-4 in formalin-fixed, paraffin-embedded tissue from the 7 fetuses positive for BoHV-4 by PCR, ISH testing proved that all 7 were infected with BoHV-4 (Table I). Nucleic acid of BoHV-4 was clearly vizualized, with negligible background staining. Viral DNA was observed in the splenic lymphocytes and monocytes in all 7 cases, as well as in the renal tubular epithelial cells or in the hepatic Kupffer cells, in foci, in some cases (Figure 1).
Table I.
Results of histologic study and in situ DNA hybridization (ISH) of tissues from 7 aborted bovine fetuses positive by polymerase chain reaction for DNA of Bovine herpesvirus 4
| ISH results
|
||||
|---|---|---|---|---|
| Fetus | Spleen | Liver | Kidney | Histologic findings |
| 1 | + | + | − | Mild subacute serous hepatitis, mild purulent bronchitis |
| 2 | + | − | − | Mild serous lymphadenitis |
| 3 | + | + | − | Mild subacute serous hepatitis |
| 4 | + | − | + | − |
| 5 | + | − | − | − |
| 6 | + | − | − | − |
| 7 | + | − | − | − |
Figure 1.
Detection of DNA (arrows) of Bovine herpesvirus 4 (BoHV-4) by in situ hybridization in 3 aborted bovine fetuses: a) in renal tubular epithelial cells of fetus 4; (b) in splenic lymphocytes and monocytes of fetus 3, and (c) in hepatic Kupffer cells of fetus 3. Note the depletion, karyorrhexis, and karyopyknosis of the splenic lymphocytes and the karyorrhexis of the hepatocytes.
Attempts to detect specific infectious agents in cases of bovine abortion usually yield negative results, no causative agent being identified (18). This study proved the presence of BoHV-4 DNA in aborted fetal tissue, detecting it first by PCR and then localizing it to fetal tissue cells. The negative results of virus isolation and IHC tests were probably due to bacterial contamination, tissue autolysis, and the fixation process, or to the lack of appropriate monoclonal antibodies. We think that our ISH results can be considered specific: the positive signals can safely be regarded as indicators of the presence of the BoHV-4 genome, since the PCR positivity supports our hybridization data and proves the presence of BoHV-4 DNA in the fetal tissues. As a probe we used a 567-bp-long PCR product of ORF 20, which is conserved in other herpesviruses. This sequence did not show any homology with other herpesviruses (except 6.37% with Herpesvirus saimiri), so our ISH results could be considered specific for the detection of the BoHV-4 genome. With the negative controls, ISH did not result in visible colored products.
Bovine abortion of undetermined cause is frequent (18). Although the presence of BoHV-4 in cells of aborted fetuses does not prove that this virus had an etiologic role in these abortions, our results do not contradict this possibility. Although we detected BVDV antibody or fungi in the tissues of 2 fetuses, neither fetus had histologic lesions, and it is unlikely that these agents played a role in the abortion. No other pathogens were found in the 7 studied cases in spite of the use of several diagnostic methods. Using ISH, we detected BoHV-4 DNA in splenic lymphocytes and monocytes, in renal tubular epithelial cells, and in hepatic Kupffer cells but not in circulating leukocytes. By destroying fetal cells and initiating a local immune response, BoHV-4 might disturb the physiological role of the infected organs, which could cause abortion. This virus is known to cause persistent and latent infection. Its role in bovine abortion needs further study, as it seems that metritis and mastitis may not be the only clinical effects of BoHV-4 infection in cattle. Thus, although this agent has so far not been considered an important bovine pathogen, it could be the reason for considerable economic losses in the cattle industry.
Acknowledgment
We thank Ágnes Mészáros for her reliable and accurate histologic and IHC work.
References
- 1.Egyed L. Bovine herpesvirus type 4: a special herpesvirus. Acta Vet Hung. 2000;48:501–513. doi: 10.1556/004.48.2000.4.13. [DOI] [PubMed] [Google Scholar]
- 2.Goyal SM, Naeem K. Bovid herpesvirus-4: a review. Vet Bull. 1992;62:181–201. [Google Scholar]
- 3.Thiry E, Bublot M, Dubuisson J, et al. Molecular biology of bovine herpesvirus type 4. Vet Microbiol. 1992;33:79–92. doi: 10.1016/0378-1135(92)90037-t. [DOI] [PubMed] [Google Scholar]
- 4.Kirkbride CA. Viral agents and associated lesions detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Invest. 1992;4:374–379. doi: 10.1177/104063879200400402. [DOI] [PubMed] [Google Scholar]
- 5.Frazier K, Pence M, Mauel MJ, et al. Endometritis in post-parturient cattle associated with bovine herpesvirus-4 infection: 15 cases. J Vet Diagn Invest. 2001;13:502–508. doi: 10.1177/104063870101300608. [DOI] [PubMed] [Google Scholar]
- 6.Smith KC. Herpesviral abortion in domestic animals. Vet J. 1997;153:253–268. doi: 10.1016/s1090-0233(97)80061-5. [DOI] [PubMed] [Google Scholar]
- 7.Parks JB, Kendrick JW. The isolation and partial characterization of a herpesvirus from a case of bovine metritis. Arch Gesamte Virusforsch. 1973;41:211–215. doi: 10.1007/BF01252768. [DOI] [PubMed] [Google Scholar]
- 8.Kendrick JW, Osburn BI, Kronlund N. Pathogenicity studies on a bovine herpesvirus. Theriogenology. 1976;6:447–462. [Google Scholar]
- 9.Schiefer B. Bovine abortion associated with cytomegalovirus infection. Zentralbl Veterinarmed B. 1974;21:145–151. [PubMed] [Google Scholar]
- 10.Reed DE, Langpap TJ, Bergeland ME. Bovine abortion associated with mixed Movár 33/63 type herpesvirus and bovine viral diarrhoea virus infection. Cornell Vet. 1979;69:54–66. [PubMed] [Google Scholar]
- 11.Castrucci G, Frigeri F, Cilli V, et al. A study of herpesvirus isolated from dairy cattle with a history of reproductive disorders. Comp Immunol Microbiol Infect Dis. 1986;9:3–21. doi: 10.1016/0147-9571(86)90070-6. [DOI] [PubMed] [Google Scholar]
- 12.Czaplicki G, Thiry E. An association exists between bovine herpesvirus-4 seropositivity and abortion in cows. Prev Vet Med. 1998;33:235–240. doi: 10.1016/s0167-5877(97)00036-6. [DOI] [PubMed] [Google Scholar]
- 13.Naeem K, Murtaugh MP, Goyal SM. Tissue distribution of bovid herpesvirus-4 in inoculated rabbits and its detection by DNA hybridization and polymerase chain reaction. Arch Virol. 1991;119:239–255. doi: 10.1007/BF01310673. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Russel DW. Molecular Cloning. A Laboratory Manual. 3rd ed. Volume 1. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 2001:6.9.
- 15.Egyed L, Ballagi-Pordány A, Bartha A, Belák S. Studies of in vivo distribution of bovine herpesvirus type 4 in the natural host. J Clin Microbiol. 1996;34:1091–1095. doi: 10.1128/jcm.34.5.1091-1095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellenberg GJ, Verstraten ER, Belák S, et al. Detection of bovine herpesvirus 4 glycoprotein B and thymidine kinase DNA by PCR assays in bovine milk. J Virol Methods. 2001;97:101–112. doi: 10.1016/s0166-0934(01)00341-x. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzacher T, Heslop-Harrison P. Practical in situ Hybridization. Oxford, England: Bios Scientific Publishers, 2000.
- 18.Kirkbride CA. Etiological agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Invest. 1992;4:175–180. doi: 10.1177/104063879200400210. [DOI] [PubMed] [Google Scholar]